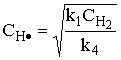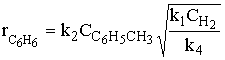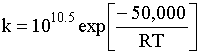Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Chapter 9 Self Test
Pseudo Steady State Hypothesis
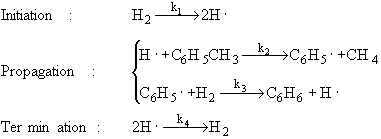
1) An example of a gas phase chain reaction involving free radicals is hydrodealkylation, in which alkyl aromatics are converted to unsubstituted aromatics. A process for the hydrodealkylation of toluene from petroleum stocks to produce benzene and methane has been developed. The hydrodealkylation occurs in the gas phase at high temperature and involves free radical. The free radical mechanism is believed to proceed by the sequence |
|
|
The specific reaction rates k1 and k4 are defined w.r.t. H2. |
Derive the reaction rate law for the rate of formation of benzene based on this mechanism. |
Hint: the reaction is 1/2 order in H2 and 1st order in toluene. |
Solution
|
|
|
For the elementary equation, we write the rate of formation of benzene as:
|
|
|
|
(1) |
|
We need to eliminate the concentration of the free radical
|
|
|
|
(2) |
|
|
(3) |
|
Adding equations 2 and 3 yield
|
|
|
|
(4) |
|
Solving for
|
|
|
|
(5) |
|
Solving for equation 3 for
|
|
|
|
(6) |
|
We can now eliminate
|
|
|
|
(7) |
|
|
(8) |
| where | |
|
|
(9) |
|
|
|