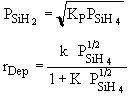Chapter 10: Catalysis and Catalytic Reactors
For the homogeneous reaction:
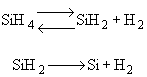 |
||
|
We found that |
|
|
|
The first reaction is in equilibrium |
|
|
| |
Case A: Small Consumption of SIH2 |
| In many cases a very thin film of the material is deposited
(a few molecular layers) and as a result very little SiH2 is
consumed. For example if SiH4 dissociates to form 1 mole SiH2
and 1 mole of H2 at equilibrium and then 0.01 mole of SiH2
is consumed in the deposition, then for all practical purposes |
|
|
| Case B: Significant Consumption of SIH2 |
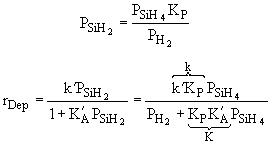
| One the other hand if significant amounts of SiH2
are consumed, we can still obtain |
|
|
| which is the same as Eqn. below (S10-12) on p666. |
|
|