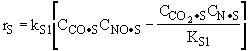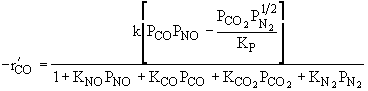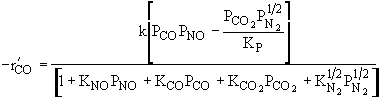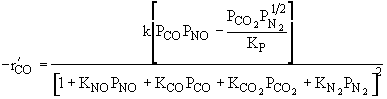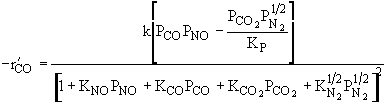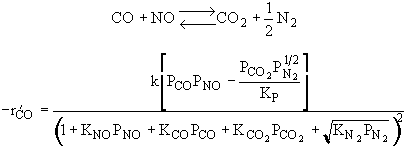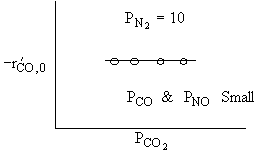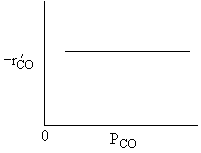Chapter 10: Catalysis and Catalytic Reactors
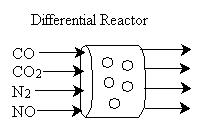
CO/NO Problem
1)|
|
||||||||||
|
Mechanism |
||||||||||
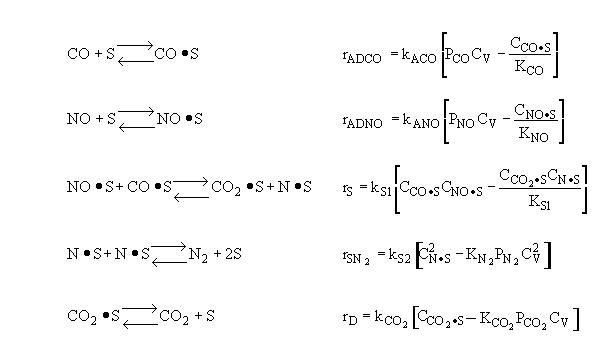
|
||||||||||
|
Surface Reaction Limits |
||||||||||
|
|
||||||||||
| Which of the following is false: |
||||||||||
|
|
||||||||||
2)
|
|
|||||||||||
Which of the following rate laws is correct |
|||||||||||
|
|||||||||||
3)
| |
|
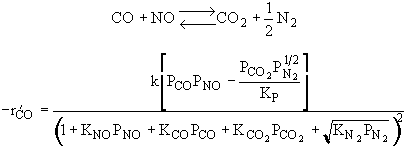
|
|
|
|
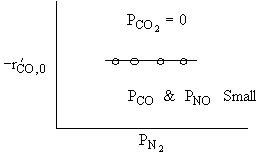
|
|
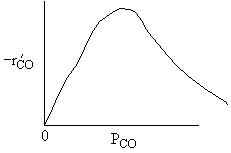
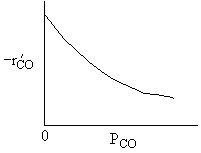
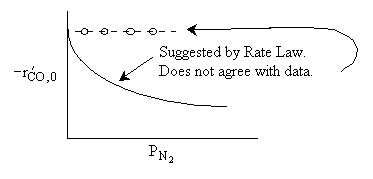
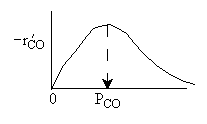
What can you tell from the above figure? |
|
|
4)
|
|
|
|
|
|
|
|
| Which of the following are true? |
|
|
|
5)
|
|
||||||||||||
|
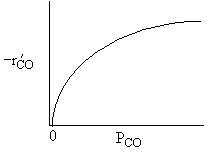
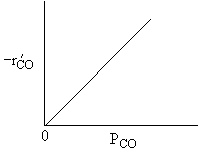
For a fixed concentration of NO which of the following curves describes the rate law for the case when PNO is very very small?
|
A is True |
||
|
Similarly, |
||
B is True |
||
D is True |
||
But, |
||
E is True |
||
|
The rate law is |
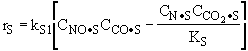
|
and |
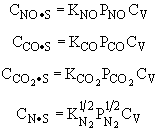
|
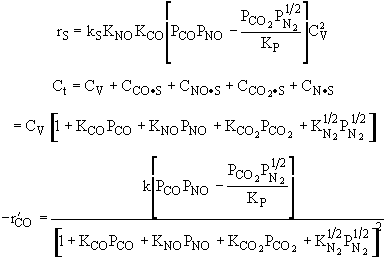
Substituting for |
|
|
|
|
|
|
|
|
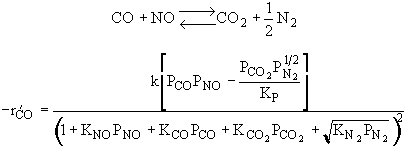
|
|
|
Rate law suggests |
|
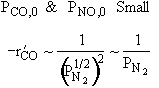
|
|
|
|
Ans: B, N2 weakly adsorbed |
|
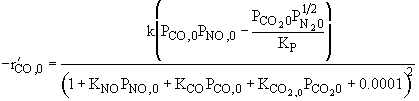
|
1)
Reaction is irreversible. |
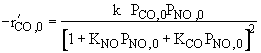
|
1 and 2 are true, all others are false. |
| Low Partial Pressure CO | |
|
|
|
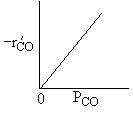
|
|
| High Partial Pressure CO | |
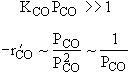
|
|
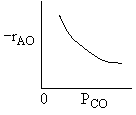
|
|
| Combining | |
|
|
| Ans: D Differentiating the rate law in with respect PCO to find the maximum |
|
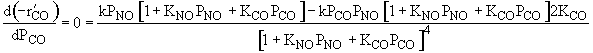
|
|
| Solving | |