Chapter 12: Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Variable Coolant Temperature - Living Example Problem
Load the Polymath program from the web. Run the Polymath program and fill in the sketches below. No solution will be given on the web or disk. You will need to describe the trends yourself after running the program.
Exothermic reversible reaction with a variable coolant temperature. The elementary reaction
![]() is carried out in a PBR that has a heat exchanger surrounding the reactor. The feed is equal molar in A and B with FAo = 5 mol/s. The coolant surrounding the PBR flow in the same direction as the reactant. The feed enters at a temperature of 325K. Plot X, Xe and T as a function of catalyst weight up to W = 4500 kg, for the base case given below
is carried out in a PBR that has a heat exchanger surrounding the reactor. The feed is equal molar in A and B with FAo = 5 mol/s. The coolant surrounding the PBR flow in the same direction as the reactant. The feed enters at a temperature of 325K. Plot X, Xe and T as a function of catalyst weight up to W = 4500 kg, for the base case given below
Additional Information (Base Case)
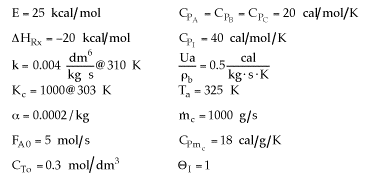 Vary the following parameters and write a paragraph describing the trends you find for each parameter variation and why they work the way they do. Use the base case for parameters not varied.
Vary the following parameters and write a paragraph describing the trends you find for each parameter variation and why they work the way they do. Use the base case for parameters not varied.
(a) Make a plot of T and X versus W for the base case given above. Compare it to the following sketches
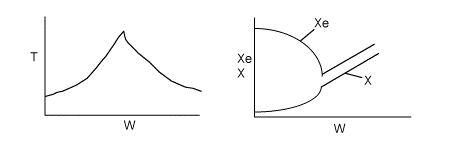 (b) On the figure below sketch T vs. W for values of FAo above and below the base case in the range (1 < FAo < 8).
(b) On the figure below sketch T vs. W for values of FAo above and below the base case in the range (1 < FAo < 8).
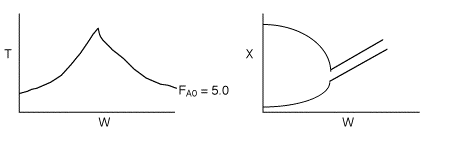 (c) On the figure below sketch T, Xe, and X for values of
(c) On the figure below sketch T, Xe, and X for values of![]() above and below the base in the range
above and below the base in the range![]() (0.5 <
(0.5 <![]() < 4).
*Note: The program gives
< 4).
*Note: The program gives![]() =1.0. Therefore, when you vary
=1.0. Therefore, when you vary![]() , you will need to account for the corresponding increase or decrease of CAo since the total concentration, CTo, is constant.
, you will need to account for the corresponding increase or decrease of CAo since the total concentration, CTo, is constant.
 (d) On the figure below sketch T, Xe, and X versus W values of above and below the base case in the range
(d) On the figure below sketch T, Xe, and X versus W values of above and below the base case in the range![]()
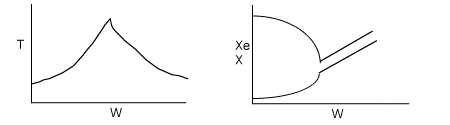 (e) On the figure below sketch T, Xe, and X vs. W for values of To above and below the base case in the range (310 < To < 350).
(e) On the figure below sketch T, Xe, and X vs. W for values of To above and below the base case in the range (310 < To < 350).
 (f) On the figure below sketch T, Xe and X vs. W for values of Ta above and below the base case in the range 300 < Ta < 340.
(f) On the figure below sketch T, Xe and X vs. W for values of Ta above and below the base case in the range 300 < Ta < 340.
 (g) On the figure below sketch T vs. W for values of
(g) On the figure below sketch T vs. W for values of![]() above and below the base case in the range
above and below the base case in the range![]() . The entering coolant temperature, Ta, is 320 K and the entering temperature of the reactants is 330 K. Also vary Ta and To .
. The entering coolant temperature, Ta, is 320 K and the entering temperature of the reactants is 330 K. Also vary Ta and To .
 (h) Repeat (g) for To = 350 and Ta = 300. Determine the conversion in a 5000 kg fluidized CSTR where
(h) Repeat (g) for To = 350 and Ta = 300. Determine the conversion in a 5000 kg fluidized CSTR where![]()
(i) If the reaction were endothermic with KC = 0.01 at 303 and![]() . Make a plot of T and X versus W for the base case given above. Compare it to the following sketches
. Make a plot of T and X versus W for the base case given above. Compare it to the following sketches
