Chapter 13: Unsteady State Nonisothermal Reactor Design
Example DVD13-2: Startup of a CSTR
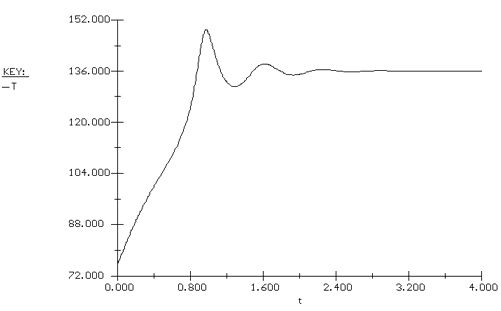
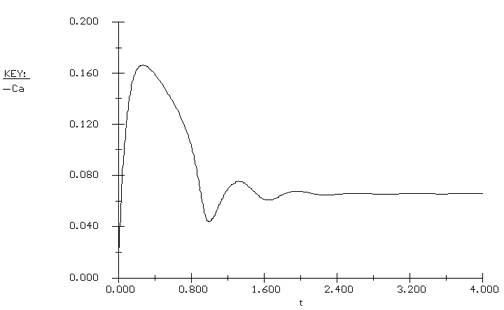
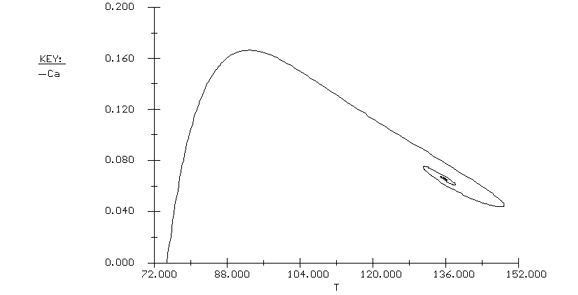
Again we consider the production of propylene glycol (C) in a CSTR with a heat exchanger. Initially there is only water at 75°F and 0.1 wt % H2SO4 in the 1420-gallon reactor. The feed stream consists of 400 lb mol/h of propylene oxide (A), 5000 lb mol/h of water (B) containing 0.1 wt % H2SO4 ,and 20 lb mol /h of methanol (M). Plot the temperature and concentration of propylene oxide as a function of time, and a concentration vs. temperature graph for different entering temperatures. |
|||
Solution |
|||
|
|
|||
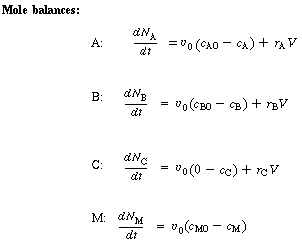
|
|
||
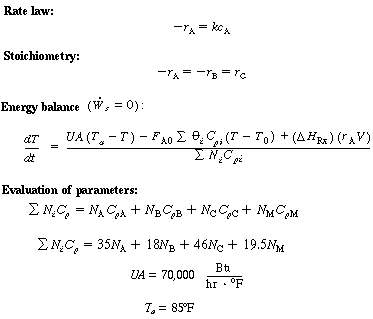
|
|
||
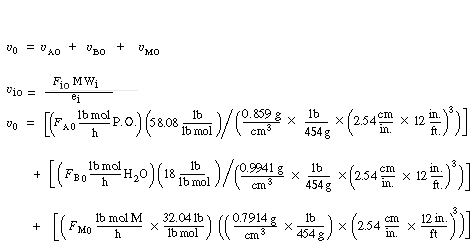
|
(CDE13-2.9) |
||
|
|||
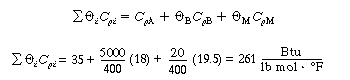
|
(CDE13-2.10) |
||
Neglecting |
|||
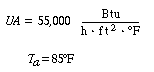
|
|||
The POLYMATH program is shown in Table CDE13-2.1. Figures CDE13-2.1 and CDE13-2.2 show the reactor temperature and concentration of propylene oxide as a function of time. One observes that both the concentration of A and the temperature oscillate around their steady-state values before coming to rest at these values. The corresponding phase-plane plot of concentration and temperature shows a spiral approach to the steady state (Figure CDE13-2.3). Table CDE13-2.1
|