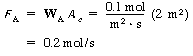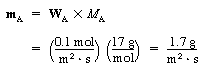Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Learning Resources
Example CD14-1: Calculating Steady-State Fluxes
Determine the molar flux of ammonia, WA,and the mass flux of ammonia, mA,through the stationary frame at steady state for the system shown in Figure CDE11-1.1. Ammonia is diffusing at steady state from point 1 to point 2. There are 12.04 x 1023 molecules of ammonia that pass through an area bounded by a stationary frame with a dimension of 1 m x 2 m in the direction of the lower concentration and over a time interval of 10 s. |
|||
SolutionThe flux is the flow rate per unit area normal to the direction of flow. The number of moles of ammonia crossing the boundary in the 10-s interval is |
|||
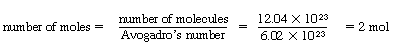
|
|||
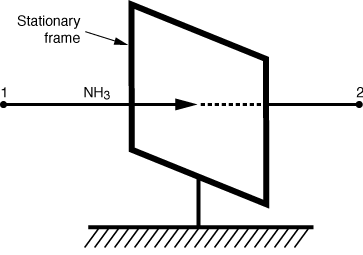
Figure CDE11-1.1 |
|||
Two moles pass through an area of 2 m2 in 10 s. Therefore, the number of moles passing through 1 m2 per second is |
|||
|
|
(CDE11-1.1) |
||
The molar flow rate is |
|||
|
|
(CDE11-1.2) |
||
The mass flux of A is the molar flux of A times the molecular weight of A, MA: |
|||
|
|
(CDE11-1.3) |
||