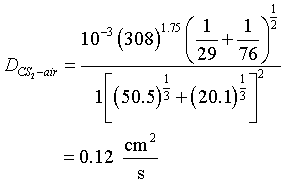Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Example 11.1: Prediction of Binary Gas Diffusivities
Fuller, Schettler, and Giddings Correlation
One of the most common equations used in predicting binary gas diffusivities owing to its theoretical foundations, is the Hirschfelder-Bird-Spotz equation†. A more recent empirical correlation has been developed by Fuller‡. Fuller used 308 experimental values of the diffusivities of various gases to determine the coefficients a, b, c, d, g, and f equation
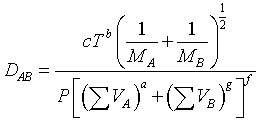
Using a nonlinear least-squares analysis, the empirical equation that gives the smallest standard deviation is
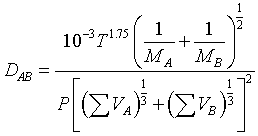
| where | P = | total pressure, atm |
| Mi = | molecular weight | |
| DAB = | diffusivity, cm2/s | |
| T = | temperature, K | |
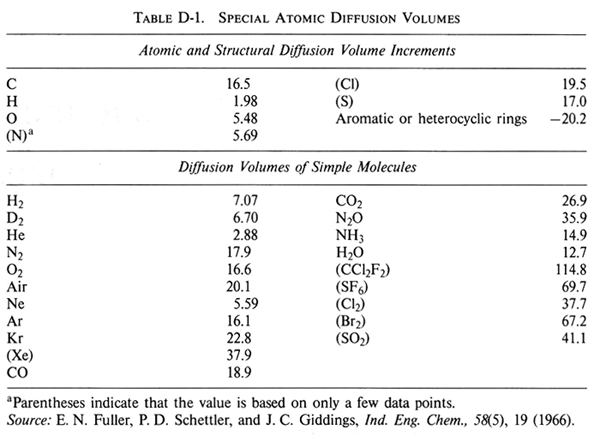
| ΣVi = | Sum of the diffusion volume for component i, as given in Table D-1 |
† See R. B. Bird, W. E. Stewart, and E. N. Lightfoot, Transport Phenomena (New York: Wiley, 1960), p. 511.
‡ E. N. Fuller, P. D. Schettler, and J. C. Giddings, Ind. Eng. Chem. 58(5), 19 (1966).
Several other equations for predicting diffusion coefficients can be found in R. C. Rcid, J. M. Prausnitz and T. K Sherwood, The Properties of Gases and Liquids 3rd ed. (New York: McGraw-Hill, 1977), Chap 11.
Example
Calculate the diffusivity of CS2 in air at 35°C at 1 atm
Solution
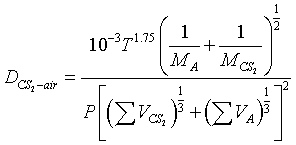
For air:
- VA = 20.1.
- 2. Molecular weight = 29.0.
For CS2, from Table D-1:
| C = 16.5 | C = 16.5 | |
| S = 17.0 | S2 = 34.0 | |
| total | 50.5 |
- VCS2 = 50.5
- Molecular weight = 76.