Chapter 15: Diffusion and Reaction in Porous Catalysts
Additional Homework Problems
CDP15-BB
The following table was obtained from the data taken in a slurry reactor for the hydrogenation of methyl linoleate to form methyl oleate.
L + H2 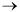 O
O
S = solubility of H2 in the liquid mixture, mol/dm3
m = catalyst charge, g/dm3
-r'L = rate of reaction of methyl linoleate, mol/dm3 min
min
| Catalyst Size | S/-r'L (min) | 1/m (dm3/g) |
|---|---|---|
| A | 4.2 | 0.01 |
| A | 7.5 | 0.02 |
| B | 1.5 | 0.01 |
| B | 2.5 | 0.03 |
| B | 3.0 | 0.04 |
- Which catalyst size has the smaller effectiveness factor?
- If catalyst size A is to be used in the reactor at a concentration of 50 g/dm3, would a significant increase in the reaction be obtained if a more efficient gas sparger were used?
- If catalyst size B is to be used, what is the minimum catalyst charge that should be used to ensure that the combined diffusional resistances for the pellet are less than 50% of the total resistance?
[3rd Ed. P12-19]