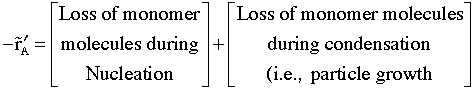
We now focus on the rate of disappearance of monomer molecules. We lose the monomer molecules by two mechanisms: Nucleation to form particles and by condensation of monomer molecules on the newly formed particles.
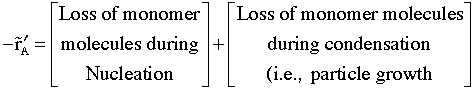
 |
The following equation is proposed for the nucleation kinetics (Girshick and Chiu, 1990). The rate of formation of stable nuclei per kg of gas is
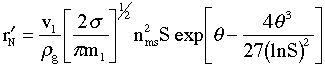 |
(# of nuclei)/kg(gas)/s | (9) |
where v1 and m1
are the molecular volume and mass of alumnimum, rg
is the gas density, nms is the saturation concentration
of aluminum molecules, S is the supersaturation ratio, q
is the dimensionless surface tension,
 and s1
is the surface area of the aluminum molecules. The saturation ratio is just
the partial pressure of monomer, P1, divided
by the vapor pressure, PV
and s1
is the surface area of the aluminum molecules. The saturation ratio is just
the partial pressure of monomer, P1, divided
by the vapor pressure, PV
|
|
The units and orders of magnitude of each of these symbols is given at the end in Table A. For the conditions given for Al, the rate of formation of stable nuclei falls in the range.
|
|
(10) |
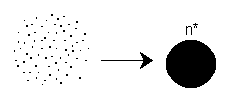
We now need to find the critical nucleus size, n*,
above which the particles are stable and can grow and below which the g-mer,
i.e. particle, is unstable. A stable particle cannot revert back to the individual
molecules where an unstable particle can. The rate of disappearance of monomer
(Al atom) by nucleation is just![]() , where n* is the critical nucleus size, i.e., number of monomer molecules per
nuclei.
, where n* is the critical nucleus size, i.e., number of monomer molecules per
nuclei.
A schematic of a g-mer of diameter dg made up of g monomers (aluminum molecules) each with diameter d1 is shown below.
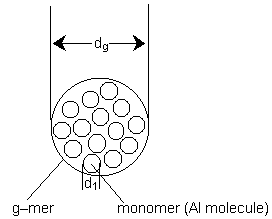 |
The volume of the g mer is
The surface area of the g-mer is
|
Figure 4. A g-mer cluster made up g monomers
We now determine the change is free energy, ![]() ,
to form a cluster of size g from g individual monomer molecules. It consists
of two terms, one to bring the dispersed monomer molecules close together,
,
to form a cluster of size g from g individual monomer molecules. It consists
of two terms, one to bring the dispersed monomer molecules close together, ![]() ,
and the other to coalesce the liquid monomer into a drop,
,
and the other to coalesce the liquid monomer into a drop, ![]() .
.
![]()
See p57 Basic Principles of Colloid Science by D. H. Everett Royal Society of Chemistry ISBN 0-85186-443-0 1988 for the details.]
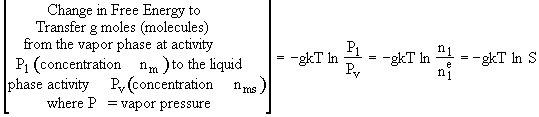 |
where
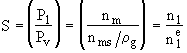 where
n1 is the concentration of monomer molecules
(molecules/dm3) and n1e
is the concentration of monomer molecules at saturation equilibrium (molecules/dm3).
where
n1 is the concentration of monomer molecules
(molecules/dm3) and n1e
is the concentration of monomer molecules at saturation equilibrium (molecules/dm3).
For

Substituting for ![]() and
and
![]()
![]()
Dividing by kT and replacing ![]() by
the dimensionless surface tension q
by
the dimensionless surface tension q
|
|
(11) |
where
![]()
![]() is shown as a function of particle
size, g, below
is shown as a function of particle
size, g, below
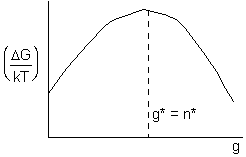
at maximum, g*=n*,
 ,
,
Solving for g*
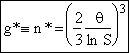 (12)
(12)
Particles of size greater than the critical cluster (i.e. particle) size g* (n*) "stable"; Generally, particles larger than n* will grow while particles small than n* are not stable and can break up.

Figure 5. Dimensionless free energy as a function of cluster size g showing the variation of critical nucleus size n* with degree of supersaturation, (see Eqn. 12).
We note that the higher the supersaturation, S, the smaller the critical nucleus
size n*
Summary for nucleation.
|
|
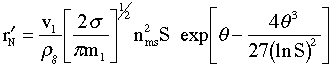 |
(# of nuclei)/kg(gas)/s | (13) |