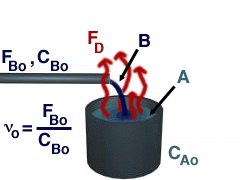
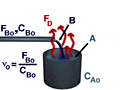
Reactive Distillation is portrayed in the picture below.

To illustrate the effects of reactive distillation lets take the
equation :
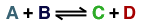
Taking the reaction to be elementary, the rate law is :
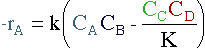
In reactive distillation, one of the products is continually removed causing the concentration of the removed product to become very small. As a result the reverse reaction decreases and more product is formed.
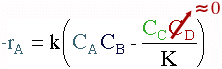
Removing a component also has an effect on the mole balance. If
D is the only component being
removed, its mole balance becomes:

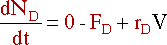 |
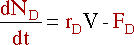 |
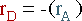 |
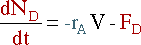 |
Again, the overall mass balance must be done. However, this time the flow of D out of the system must be taken into account.


The mass terms in the equation can be replaced by :
 |
 |
 |
||||
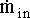 |
 |
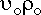 |
||||
 |
 |
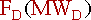 |
giving :
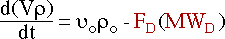
Assuming that the system is at constant density leads to :


| Mole Balance | Mass Balance | |||
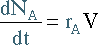 |
 |
|||
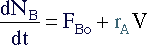 |
||||
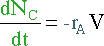 |
Rate Law | |||
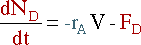 |
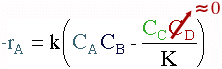 |
|||
|
|