

 You have been given a summer internship by
J. Flabetes Inc., a somewhat well known chemical company located in
sunny California. Your last project for the summer is to study a
proposed method of removing acetic acid from the waste water. The acetic
acid is to be removed by reacting it with methanol in a catalyst filled
semibatch reactor :
You have been given a summer internship by
J. Flabetes Inc., a somewhat well known chemical company located in
sunny California. Your last project for the summer is to study a
proposed method of removing acetic acid from the waste water. The acetic
acid is to be removed by reacting it with methanol in a catalyst filled
semibatch reactor :
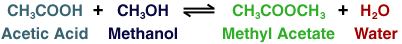
It has been suggested that reactive distillation be included in the plans, but your boss is kind of skeptical. Your job is to simulate the reaction and see if reactive distillation is a better option than without.
We will begin by looking at the semibatch reactor case with no reactive distillation.
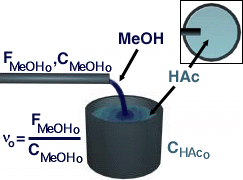
| Mole Balance | Mass Balance | |||
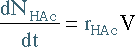 |
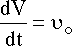 |
|||
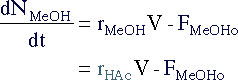 |
||||
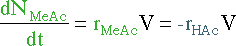 |
||||
 |
||||
| Rate Law | ||||
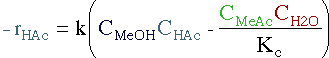 |
||||
Polymath
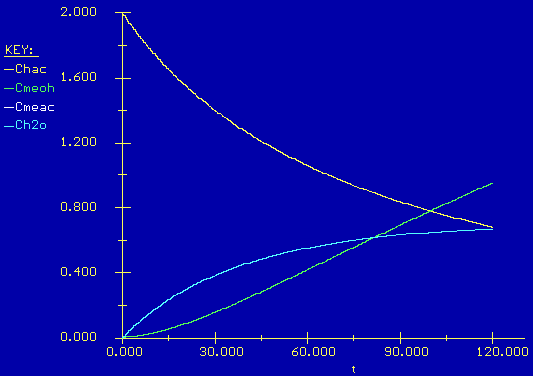 Click here for clear view of graph
Click here for clear view of graph
From the results we see that after 120 minutes, 116 moles of acetic acid is left in the reactor from the original 300. This is a conversion of 61.3 %
|
|