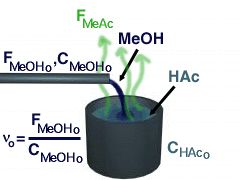
We can also use reactive distillation with the reaction and see if the conversion improves. Methyl Acetate will be the product that is removed.

| Mole Balance | Mass Balance | |||
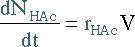 |
 |
|||
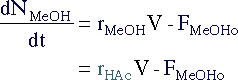 |
||||
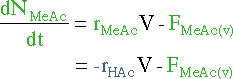 |
||||
 |
||||
| Rate Law | ||||
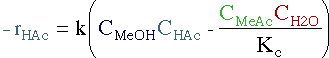 |
||||
Unfortunatly we are not given an evaporation rate for methy acetate,
so we must look else where to find one. One such equation is :
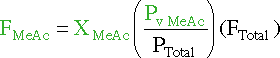
| FMeAc | Rate at which Methyl Acetate is removed from the reaction mixture by evaporation |
|
| XMeAc | Mole fraction of reaction mixture that is
methyl acetate |
|
| Pv MeAc | Vapor pressure of methyl acetate at reaction
temperature |
|
| PTotal | Total pressure of the system | |
| FTotal | Rate at which air is bubbled through the
reactor. As the species evaporate, they will be collected in the air bubbles. |
Now we need to figure out the vapor pressure of methyl acetate. From
Perry's Chemical Engineering Handbook, we can develop the equation :
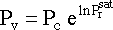
and that for methy acetate :
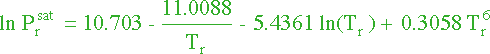
|
|