|

|
Second Coordination Sphere Effects in Copper Complexes
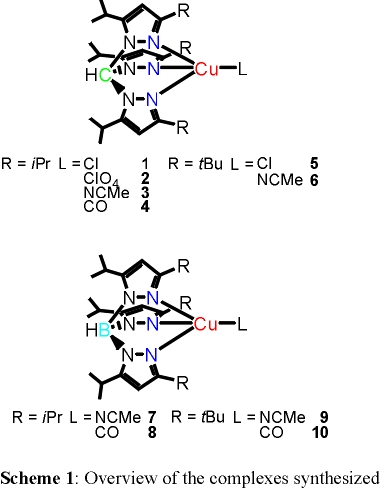
New mononuclear copper(I) complexes containing tripodal nitrogen ligands, tris(3,5-dialkyl-1-pyrazolyl)methane (Scheme 1, top) and
hydrotris(3,5-dialkyl-1-pyrazolyl)borate anion (Scheme 1, bottom), were synthesized and a number of them were structurally characterized.
Physicochemical and theoretical studies demonstrate the critical differences between the methane and borate ligands, and how the
second coordination sphere influences the reactivities of the metal centers.
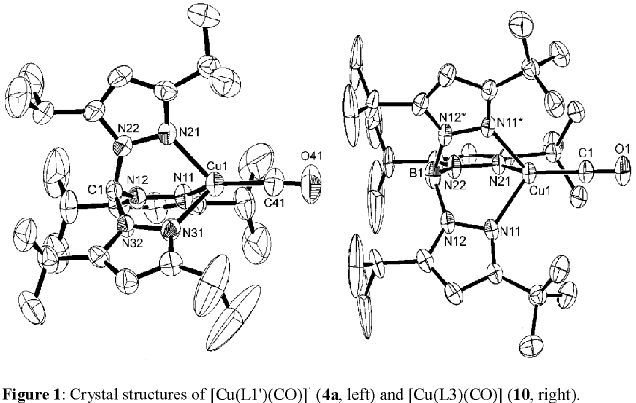
Importantly, our results show that complexes with the neutral methane and anionic borate ligands with identical substituents differ in the electron
richness of the copper(I) center, which is directly probed by the C-O stretching vibration in the corresponding carbonyl complexes. In fact, the
borate ligands lead to more electron rich copper(I) centers, in agreement with the observed redox potentials and reactivities of the complexes (with O2).
The observed trends for different alkyl substituents of the pyrazolyl rings in these ligands are less clear. Using again CO as a probe, it is demonstrated that
the observed differences between iPr and tBu substituents mostly relate to steric hindrance, whereas the electronic effect is negligible.
Finally, the influence of the size and the nature of the fourth ligand are evaluated. Based on all these results, it is possible to efficiently control
the structures, electronic characters, and reactivities of four-coordinate copper(I) complexes by variation of the charge of the ligand, the degree of
steric hindrance of the substituents, and the choice of the fourth ligand.
In proteins, copper(I) centers are observed, which either react with O2 or not, depending on their environment. Importantly, our results demonstrate how
reactivity can be controlled by the structure and charge of the ligands. In this sense, this work contributes to an understanding of how proteins are able to
control the reactivities of 'their' metal centers, and how second coordination sphere effects contribute to this.
We also investigated copper-nitrite and -NO complexes with analogous hydrotris(triazolyl)borate ligands,
in collaboration with Prof. Liz Papish (University of Alabama). These results were published in Inorg. Chem. in 2012.
Most recently, we have focused on developing biomimetic copper nitrite reductase model complexes that function as efficient electrocatalysts for the
generation of NO from nitrite. These systems will be used as on-demand NO generation platforms for applications in biomedical devices. In particular, these
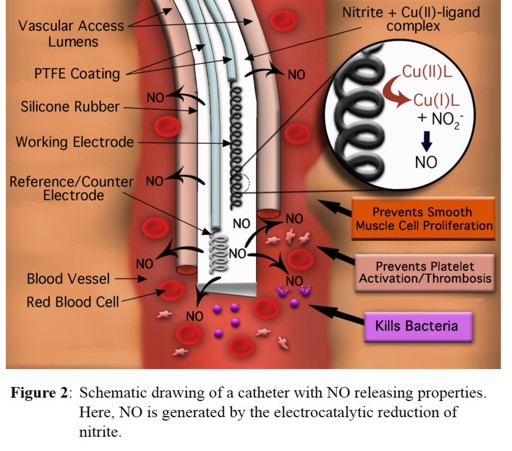
complexes will be incorporated into dual lumen catheters (in collaboration with the Meyerhoff group, University of Michigan) to generate small amounts of NO
that are released from the catheter in order to prevent blood clotting and bacterial adhesion to the catheter surface (see Figure 2). Another application of this technology
is inhalation of NO (INO) therapy, where patients breath in small amounts of NO to combat bacterial infection in the lungs (for example, in pneumonia). NO gas
cylinders of about 800 ppm of NO are required for current INO distribution systems and these cylinders are heavy, cumbersome, and very expensive. Thus,
there is a great need to develop an inexpensive yet portable source of relatively pure NO for use in INO and other biomedical applications, to make use of gas phase
NO more available to a greater number of patients, facilitating clinical trials of different gas phase NO therapies on more diseases, and ultimately making INO
available for home use (e.g., for cystic fibrosis patients) and in remote areas. Here, electrocatalytic nitrite reduction would provide an easy means
for NO generation in portable NO generators. We are therefore devolping new copper complexes that show high Faradaic efficiencies and enhanced life times for electrocatalytic
nitrite reduction for NO generation on demand.
References:
K. Fujisawa, T. Ono, Y. Ishikawa, N. Amir, Y. Miyashita, K. Okamoto, N. Lehnert
"Structural and Electronic Differences of Copper(I) Complexes with Tris(pyrazolyl)methane
and Hydrotris(pyrazolyl)borate Ligands"
Inorg. Chem. 2006, 45, 1698-1713
N. Lehnert, U. Cornelissen, F. Neese, T. Ono, Y. Noguchi, K. Okamoto, K. Fujisawa
"Synthesis and Spectroscopic Characterization of Cu(II)-Nitrito Complexes with
Hydrotris(pyrazolyl)borate and related Coligands"
Inorg. Chem. 2007, 46, 3916-3933
K. Fujisawa, Y. Noguchi, Y. Miyashita, K. Okamoto, N. Lehnert
"Mononuclear and Binuclear Copper(I) Complexes Ligated by Bis(3,5-diisopropyl-1-pyrazolyl)methane:
Insight into the Fundamental Coordination Chemistry of Three-Coordinate Copper(I) Complexes with a
Neutral Coligand"
Inorg. Chem. 2007, 46, 10607-10623
F. Paulat, N. Lehnert, Y. Ishikawa, K. Okamoto, K. Fujisawa
"Mononuclear and Binuclear Copper(I)-Diazene Complexes: a New Chapter of Copper
Coordination Chemistry"
Inorg. Chim. Acta 2008, 361, 901-915
(special issue in honor of Edward I. Solomon)
K. Fujisawa, A. Tateda, T. Ono, Y. Miyashita, K. Okamoto, F. Paulat, V. K. K. Praneeth, N. Lehnert
"Structural and Spectroscopic Characterization of Mononuclear Copper(I) Nitrosyl
Complexes: End-on versus Side-on Coordination of NO to Copper(I)"
J. Am. Chem. Soc. 2008, 130, 1205-1213
A. C. Merkle, N. Lehnert
"The Side-on Copper(I)-Nitrosyl Geometry in Copper Nitrite Reductase is Due to Steric Interactions with
Isoleucine-257"
Inorg. Chem. 2009, 48, 11504-11506
A. C. Merkle, N. Lehnert
"Binding and Activation of Nitrite and Nitric Oxide by Copper Nitrite Reductase and Corresponding Model
Complexes"
Dalton Trans. 2012, 41, 3355-3368
(invited contribution: Dalton Perspective; selected for Journal cover: Issue 12, March 28, 2012)

M. Kumar, N. A. Dixon, A. C. Merkle, M. Zeller, N. Lehnert, E. T. Papish
"Hydrotris(triazolyl)borate Complexes as Functional Models for Cu Nitrite Reductase: The Electronic Influence of
Distal Nitrogens"
Inorg. Chem. 2012, 51, 7004-7006
H. Ren, J. Wu, C. Xi, N. Lehnert, T. Major, R. H. Bartlett, M. E. Meyerhoff
"Electrochemically Modulated Nitric Oxide (NO) Releasing Biomedical Devices via Cu(II)-Tri(2-pyridylmethyl)amine
Mediated Reduction of Nitrite"
ACS Appl. Mater. Interfaces 2014, 6, 3779-3783
Y. Qin, J. Zajda, E. J. Brisbois, H. Ren, J. Toomasian, T. C. Major, A. Rojas-Pena, B. Carr, T. Johnson, J. W. Haft,
R. H. Bartlett, A. P. Hunt, N. Lehnert, M. E. Meyerhoff
"Portable Nitric Oxide (NO) Generator Based on Electrochemical Reduction of Nitrite for Potential Applications in Inhaled
NO Therapy and Cardiopulmonary Bypass Surgery"
Mol. Pharmaceutics 2017, 14, 3762-3771
K. K. Konopinska, N. J. Schmidt, A. P. Hunt, N. Lehnert, J. Wu, C. Xi, M. E. Meyerhoff
"Comparison of Copper(II)-Ligand Complexes as Mediators for Preparing Electrochemically Modulated Nitric Oxide-Releasing
Catheters"
ACS Appl. Mater. Interfaces 2018, 10, 25047-25055
A. P. Hunt, A. E. Batka, M. Hosseinzadeh, J. Gregory, H. Haque, H. Ren, M. E. Meyerhoff, N. Lehnert
"Nitric Oxide Generation On Demand for Biomedical Applications via Electrocatalytic Nitrite Reduction by Copper BMPA- and
BEPA-Carboxylate Complexes"
ACS Catal. 2019, 9, 7746-7758
|





