Single-molecule studies of amyloid-beta oligomer binding on live cells
Dr. Robin D. Johnson
Abstract
Soluble oligomers of amyloid-beta peptide have been implicated as the proximal neurotoxins in Alzheimer's disease. However, the precise stoichiometric identity of the neurotoxic aggregate(s) and the mechanisms by which these species induce neuronal dysfunction remain uncertain. Amyloid-beta (Abeta) may damage cells by binding to and interfering with membrane integral protein receptors or by directly disrupting the neuronal cell membrane, allowing Ca2+ to leak into the cell. Physiologically relevant experimentation is hindered by the low endogenous concentrations of the peptide, the metastability of Abeta oligomers, and the wide range of observed interactions between Abeta and biological membranes. Single-molecule microscopy represents one avenue for overcoming these challenges. Using this technique, we find that monomeric amyloid-beta oligomerizes at low nanomolar concentrations on exposure to physiological buffers and glass or poly-D-lysine coated surfaces. Amyloid-beta(1-40) forms larger oligomers or clusters within minutes of binding to SH-SY5Y neuroblastoma cells but induces only minor Ca2+ leakage. Abeta binds to the neurites of primary rat hippocampal cells with higher affinity. While amyloid-beta(1-40) (Abeta40) and amyloid-beta(1-42) (Abeta42) form larger oligomers than those detected on slides immediately after binding, a 1:1 mix of the two peptides results in smaller neurite-bound oligomers than those detected on-slide or for either peptide alone. On-neurite oligomer growth over time requires the presence of solution peptide. With 1 nM peptide in solution, Abeta40 oligomers do not grow over the course of 48 hours, Abeta42 oligomers grow slightly, and oligomers of a 1:1 mix grow substantially. These results are significant in light of the increased Abeta42:Abeta40 ratios correlated with familial Alzheimer's disease mutations. While the majority of neurite-bound oligomers are immobile, a small population exhibits diffusive or directed motion. Neurite-bound oligomers do not preferentially associate with synapses and bind at only slightly higher density on dendrites than on axons. These results point to a mechanism by which small Abeta oligomers bind to neurons and grow at rates dependent on local Abeta42:Abeta40 ratio and peptide concentrations, with neurotoxicity emerging at some later point in time.
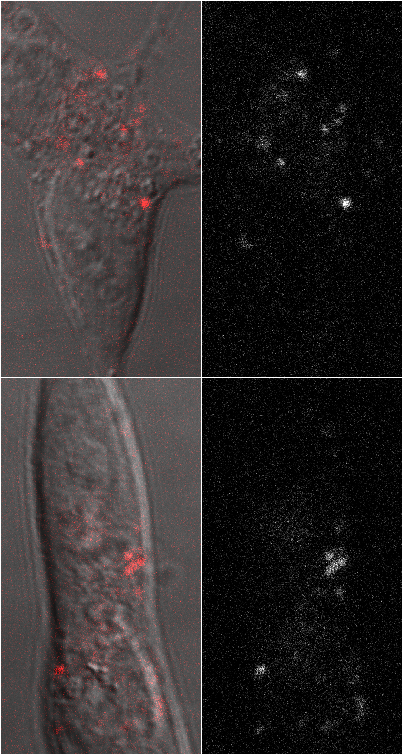
SH-SY5Y cells with single HL647AB42 oligomers
|

Primary neurons with single HL647AB40 oligomers |

Tracking AB40 oligomers on the live cell
|