

 |
||


|
||
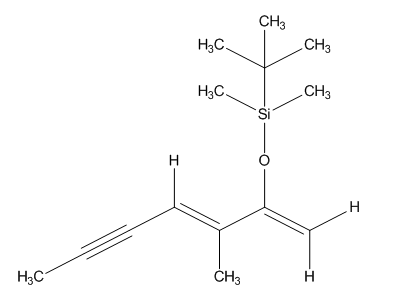
Explanations:(a): The singlet peak at 2.01 ppm corresponds to the hydrogen atoms in the methyl group at the sp center. These hydrogen atoms have 1 three bond neighbor and are the fifth most deshielded atoms because they are at a carbon located at an sp center. The semi-close proximinty to an sp center draws electrons from the hydrogen atoms somewhat strongly, deshielding them.(b): The multiplet peak at 5.88 ppm corresponds to the single hydrogen next to the triple bond. This hydrogen atom has no three bond neighbors and is the most deshielded atom because it is at an sp2 center adjacent to an sp center. Both centers, working cohesively, severely draw away electrons from the hydrogen atom, deshielding it greatly.(c): The doublet peak at 2.05 ppm corresponds to the hydrogen atoms in the methyl group at the sp2 center. These hydrogen atoms have no three bond neighbors and are the fourth most deshielded atoms because they are at a carbon located at an sp2 center close to a highly electronegative oxygen atom. Both the semi-close proximity to an sp2 center and highly electronegative oxygen atom draw electrons from the hydrogen atoms, deshielding them.(d): The peak at 4.36 ppm corresponds to the lower hydrogen in the sp2 center. This hydrogen has 1 two bond neighbor and is the third most deshielded atom because it is at an sp2 center close to a highly electronegative oxygen atom. Both the sp2 center and highly electronegative oxygen atom draw electrons from the hydrogen atom, deshielding it greatly; the deshielding effect is not as great as hydrogen (e) because this hydrogen atom is slightly farther away from the electronegative oxygen atom.(e): The doublet peak at 4.54 ppm corresponds to the upper hydrogen in the sp2 center. This hydrogen has 1 two bond neighbor and is the second most deshielded atom because it is at an sp2 center close to a highly electronegative oxygen atom. Both the sp2 center and highly electronegative oxygen atom draw electrons from the hydrogen atom, deshielding it greatly.(g): The singlet peak at 0.97 ppm corresponds to the hydrogen atoms in the methyl groups at the tertiary carbon. These hydrogen atoms have no three bond neighbors and are the second least deshielded atoms because the carbon atoms that the hydrogen atoms are attached to are equally electronegative to the tertiary carbon. This results in minimal deshielding as the carbon atoms the hydrogen atoms are attached to draw in electrons, somewhat deshielding the hydrogen atoms.(f): The singlet peak at 0.18 ppm corresponds to the hydrogen atoms in the methyl groups at the silicon atom. These hydrogen atoms have no three bond neighbors and are the least deshielded atoms because the carbon atoms that the hydrogen atoms are attached to are more electronegative than silicon. This draws in electrons toward the hydrogen atoms which increases the shielding effect. |