

Kong, K.; Moussa, Z.; Romo, D. Org. Lett. 2005, 7, 5127-5130.
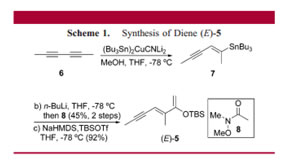
"In Ke Kong's journal article, Kong explains his ambition to discover an enantiosynthesis of the spirocyclic imine (-)-gymnodimine (a marine toxin) in order to provide modern antigens to monitor and eliminate it. Preliminary trials to promote this Diels-Alder enantioselective reaction failed with the diene (Z)-silylenol ether due to a lack of reactivity, even in the presence of chiral catalysts. This failed reactivity was most likely attributed to geometrical issues so the synthesis of the diene (E)-silylenol ether (which is our product 24) was pursued. In the first step for (E)-silylenol ether's synthesis, (E)-vinyl stannane was used as a starting material rather than other radical and metal-catalyzed hydrostannylations because its regioselectivity was more stable and therefore formed the final (E)- silylenol ether. (E)-vinyl stannane goes through tin-lithium exchange with n-BuLi followed by a reaction with the amide N-methyl-N-methoxyacetamide in order to form the ketone (which is our intermediate S-6). This essentially describes the first step for the formation of (E)- silylenol ether; the ketone continues through enolization and silyation to form (E)- silylenol ether.
Comparing References - 3 articles that cite the above article:
Toumieux, S.; Beniazza, R.; Desvergnes, V.; Araoz, R.; Molgo, J.; Landais, Y. Org. Biomol. Chem. 2011, 9, 3726-3732.
This article cited the article by Kong as a preview to their report. This article’s research consists of reporting a straightforward approach to the tetrahydrofuran core, gymnodimine, using the Ueno-Stork reaction as a key-step, but cites the first article in order to show that other approaches of synthesizing gymnodimine have already been reported.
Kong, K.; Romo, D.; Lee, C. Angew. Chem. Int. Ed. 2009, 48, 7402-7405.
This article was further research into the synthesis of (-)-Gymnodimine, the marine toxin that was being researched in the previous article. While the 2005 article only managed to synthesize a spirocyclic imine intermediate, a full synthesis for the compound was proposed in this article.
Reymond, S.; Cossy, J. Chem Rev. 2008, 108, 5359-5406.
In this article, research was done into the yields and diastereoselectivity of various copper catalyzed Diels-Alder reactions. The article refers to the Diels-Alder reaction of the six-membered cyclic imide and diene catalyzed by Cu(SbF6)2 that is used in the Kong article with high diastereoselectivity and yield.
3 articles that cited the above:
1) Wolf C.; Xu H. Chem. Commun. 2011, 47, 3319-3350.
2) Ma, M.; Lu, R.; Pullarkat, S. A.; Deng, W.; Leung, P. Dalton Trans. 2010, 39, 5453-5461.
3) Jones, A. L.; Snyder, J. K. Org. Letter. 2010, 12, 1592-1595.



