
How are Weinreb amides used in Organic Synthesis?
Weinreb ketone synthesis is a technique, developed in 1981 by Steven M. Weinreb, which is used to produce new carbon-carbon bonds in the synthesis of a ketone. In the original reaction, the “Weinreb amide”, a N-methyl-N-methoxyacetamide, was synthesized from an acid chloride, and then treated with an organometallic reagent, giving the ketone. A Grignard reagent is commonly used, or, like in the case of our synthesis, an organolithium reagent. Weinreb amides can also be reduced using lithium aluminum hydride to give the corresponding aldehyde. In each case, an acid work-up is required to give the wanted product.
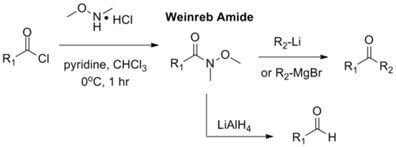
This method is preferred over the reactions of esters or carboxylic acid with organometallic reagents because the intermediate ketones from these reactions are still highly reactive and tend to react with two equivalents of the reagent to produce the alcohol. This is not a problem with the Weinreb amides and the ketone can be produced in high yield, making this a favored form of synthesis.



