

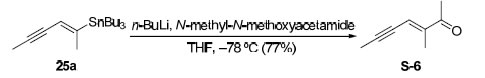
To a solution of vinyl stannane 25a (7.08 g, 19.2 mmol) in THF (150 mL) at –78 ºC was added n-BuLi (2.0 M in hexanes, 12.0 mL, 24.0 mmol). After stirring at –78 ºC for 20 min, N-methoxy-N-methyl acetamide (2.4 g, 23.0 mmol) in THF (15 mL) was added dropwise. The resulting light yellow solution was stirred at –78 ºC for 1 h, warmed to room temperature and, quenched by 1 N HCl solution (about 40 mL) until the mixture was slightly acidic. The aqueous layer was extracted with Et2O and the combined organic layers were dried (MgSO4), concentrated in vacuo, and purified by flash chromatography (5% Et2O/pentane →10% Et2O/pentane) to afford 1.8 g (77% yield) of the desired product as a yellow oil. Rf = 0.43 (5% EtOAc/hexanes)
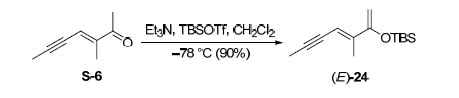
Et3N (1.47 mL, 10.5 mmol) and TBSOTf(1.31 mL, 5.79 mmol) were added to a solution of a ketone intermediate (643 mg, 5.25 mmol) in CH2Cl2 (30 mL) at –78 ºC. The reaction mixture was stirred at –78 ºC for 10 min and quenched by pH 7 buffer. The aqueous layer was extracted with CH2Cl2and the combined organic layers were dried (Na2SO4) and concentrated in vacuo. The residue was purified by flash chromatography on basic Al2O3 (pentane) to afford 1.12 g (90% yield) of the desired product as a colorless oil. Rf = 0.57 (hexanes).



