Experimental
(4aRS,8SR)-8-Chloro-4,4-dimethyl-7-methylene-3,4,5,6,7,8-hexahydro-4a,8-methanobenzo[7]-annulene 2,9-dione (19). To a solution of chloro TBS-enol ether 3 (21.0 mg, 47 µmol) dissolved in dry acetone (0.75 mL) at ambient temperature was added (acetonitrile)[(2-biphenyl) di-tert-butylphosphine]gold(I) hexafluoroabtimonate (18.0 mg, 23 µmol, 0.5 equiv). The resulting reaction mixture was tightly sealed with a polytetrafluoroethylene coated glass-stopper before it was warmed to 45 °C and stirred for 6 h between 45-50 °C. The mixture was filtered through a short plug of silica gel (2.5 cm) eluting with diethyl ether. Purification by column chromatography (EtOAc/hexane 20:80) afforded (4aRS,8SR) - 8-chloro-4,4-dimethyl-7-methylene-3,4,5,6,7,8-hexahydro-4a,8-methanobenzo-[7]-annulene-2,9-dione 19 (8.0 mg, 65%) together with ca. 2 wt % inseparable (2-biphenyl)di-tert-butylphosphine.
TLC: Rf = 0.30 (EtOAc/hexane, 20:80; UV, KMnO4);
1H-NMR (400 MHz, CDCl3): δ 6.61 (d, J = 0.8 Hz, 1H), 5.42 (d, J = 2.3 Hz, 1H), 5.01 (d, J = 2.3 Hz, 1H), 2.76-2.65 (m, 2H), 2.40 (dd, J = 11.5, 2.9 Hz, 1H), 2.30-2.09 (m, 4H), 1.95 (ddd, J = 13.4, 12.3, 5.9 Hz, 1H), 1.09 (s, 3H), 1.02 (s, 3H;
13C-NMR (101 MHz, CDCl3): δ 199.2, 198.7, 154.3, 143.0, 125.0, 110.1, 75.9, 49.2, 48.1, 45.9, 38.7, 33.1, 30.5, 24.3, 24.2;
IR (thin film): 2966, 2950, 2874, 2848, 1746, 1686, 1680, 1650, 1641, 1468, 1462, 1444, 1391, 1372, 1306, 1284, 1250, 1221, 1196, 1166, 1132, 1092, 1024, 998, 982, 944, 948, 914, 853, 742, 711 cm-1;
HRMS (EI): exact mass calculated for C15H17(35Cl)O2 [M+], 264.0912 (100% rel. abundance); found 264.0908 (100% rel. abundance); exact mass calculated for C15H17(37Cl)O2 [M+], 266.0883 (31.98% rel. abundance); found 266.0884 (33% rel. abundance).
Note: The experimental came, in full, from the supplemental information to the original paper. It should be noted, however, that “hexafluoroabtimonate” can be presumed to be a typo; the actual reagent (and the reagent that commonly accompanies gold catalysis of this type) was likely hexafluoroantimonate. No reagent “hexafluoroabtimonate” could be found to exist.
TLC: Rf = 0.30 (EtOAc/hexane, 20:80; UV, KMnO4);
1H-NMR (400 MHz, CDCl3): δ 6.61 (d, J = 0.8 Hz, 1H), 5.42 (d, J = 2.3 Hz, 1H), 5.01 (d, J = 2.3 Hz, 1H), 2.76-2.65 (m, 2H), 2.40 (dd, J = 11.5, 2.9 Hz, 1H), 2.30-2.09 (m, 4H), 1.95 (ddd, J = 13.4, 12.3, 5.9 Hz, 1H), 1.09 (s, 3H), 1.02 (s, 3H;
13C-NMR (101 MHz, CDCl3): δ 199.2, 198.7, 154.3, 143.0, 125.0, 110.1, 75.9, 49.2, 48.1, 45.9, 38.7, 33.1, 30.5, 24.3, 24.2;
IR (thin film): 2966, 2950, 2874, 2848, 1746, 1686, 1680, 1650, 1641, 1468, 1462, 1444, 1391, 1372, 1306, 1284, 1250, 1221, 1196, 1166, 1132, 1092, 1024, 998, 982, 944, 948, 914, 853, 742, 711 cm-1;
HRMS (EI): exact mass calculated for C15H17(35Cl)O2 [M+], 264.0912 (100% rel. abundance); found 264.0908 (100% rel. abundance); exact mass calculated for C15H17(37Cl)O2 [M+], 266.0883 (31.98% rel. abundance); found 266.0884 (33% rel. abundance).
Note: The experimental came, in full, from the supplemental information to the original paper. It should be noted, however, that “hexafluoroabtimonate” can be presumed to be a typo; the actual reagent (and the reagent that commonly accompanies gold catalysis of this type) was likely hexafluoroantimonate. No reagent “hexafluoroabtimonate” could be found to exist.
Huwyler, N.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, 13066 – 13069.
Huwyler, N.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, S. I. 1-61.
Huwyler, N.; Carreira, E. M. Angew. Chem. Int. Ed. 2012, 51, S. I. 1-61.
Glossary
Eluting: The process of extracting or separating one material from another, usually by washing with solvent. The “eluting agent” is the solvent used to do this washing or separation.
Purification: The physical separation of a chemical of interest from foreign or contaminating substances. Can be done through various methods; such as heating, separatory funnel, column chromatography, etc.
Column Chromatography: A frequently employed method of purification in chemistry. The process of separation is as follows: A mixture of chemicals is placed onto a column of stationary phase (often silica gel.) The mixture is run through the column with the use of a mobile phase, a solvent. The degree of hydrogen bonding with the silica gel and with the solvent both contribute to each compound present in the mixture moving faster or slower through the column. Due to the large amount of hydrogen bonding that silicon is capable of, compounds with more hydrogen bonding ability will move more slowly through the column, and a more hydrogen bond-able eluting agent will cause compounds to move faster through the column. This phenomenon can be seen in the figure below.
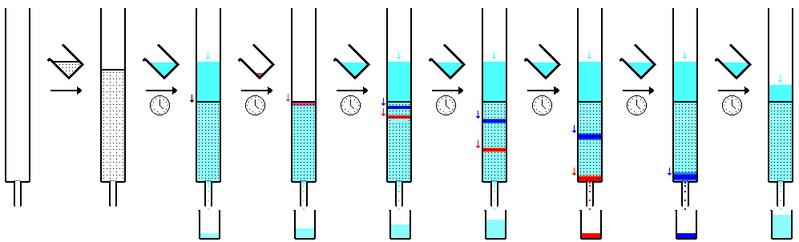
In this diagram the blue layer has stronger interactions with the silica gel, causing it to move through the column more slowly. This allows the mixture to separate completely and come out of the column as pure substances.
Wikimedia Commons. http://upload.wikimedia.org/wikipedia/commons/3/3f/Column_chromatography_sequence.png (accessed: April 2, 2013).
Eluting: The process of extracting or separating one material from another, usually by washing with solvent. The “eluting agent” is the solvent used to do this washing or separation.
Purification: The physical separation of a chemical of interest from foreign or contaminating substances. Can be done through various methods; such as heating, separatory funnel, column chromatography, etc.
Column Chromatography: A frequently employed method of purification in chemistry. The process of separation is as follows: A mixture of chemicals is placed onto a column of stationary phase (often silica gel.) The mixture is run through the column with the use of a mobile phase, a solvent. The degree of hydrogen bonding with the silica gel and with the solvent both contribute to each compound present in the mixture moving faster or slower through the column. Due to the large amount of hydrogen bonding that silicon is capable of, compounds with more hydrogen bonding ability will move more slowly through the column, and a more hydrogen bond-able eluting agent will cause compounds to move faster through the column. This phenomenon can be seen in the figure below.

In this diagram the blue layer has stronger interactions with the silica gel, causing it to move through the column more slowly. This allows the mixture to separate completely and come out of the column as pure substances.
Wikimedia Commons. http://upload.wikimedia.org/wikipedia/commons/3/3f/Column_chromatography_sequence.png (accessed: April 2, 2013).