References
The paper (cited below) covers gold (I) catalysis and the formation of a ring with the loss of a silicon group and a decrease in bond order: all of these things occur in our paper, which immediately makes it likely to be relevant. Upon further investigation, we see that this paper proposes the addition of gold to the multiple bond, followed by protonolysis. Ultimately, we determined that a similar mechanism occurred in our paper, although in the transformation from 3 to 19 two silyl groups are lost instead of just one. In addition, this paper confronts one of the major problems we had in our reaction, which was the exact source of the protons for protonolysis. Herein they point out that the counter-ion for gold(I) is important for this, and both their papers and ours uses a polyphenyl phosphine reagent, suggesting further similarity there. We discovered from this paper that the lack of a simple strong acid is that if a strong acid is present, it will competitively react with the silyl group, and undesirable side products could therefore occur if the acid is too strong.
Staben, S. T.; Kennedy-Smith, J. J.; Huang, D.; Corkey, B. K.; LaLonde, R. L.; Toste, F. D. Angew. Chem. Int. Ed. 2006, 118, 6137–6140.
Papers that cited the above paper:
Duschek, A.; Kirsch, S. F. Angew. Chem. Int. Ed. 2008, 47, 5703 – 5705.

This is relevant to the initial paper because of the similar use of a triphenyl gold catalyst, and the creation of a ring and an alkene from an alkyne.
Fürstner, A.; Davies, P. W. Angew. Chem. Int. Ed. 2007, 46, 3410 – 3449.
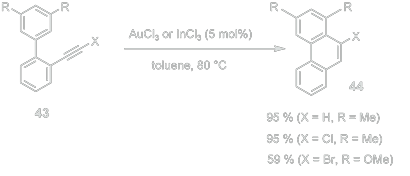
This is relevant to the initial paper because of the similar (optional) use of a gold catalyst, as well as the similar ring formation.
Buzas, A. K.; Istrate, F. M.; Gagosz, F. Angew. Chem. Int. Ed. 2007, 119, 1159 –1162.
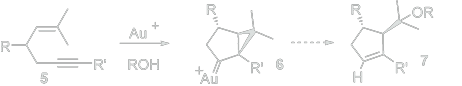
This is relevant to the initial paper because of the similar use of a gold(I) catalyst, as well as, once again, the creation of a ring and an alkene from an alkyne
Photo Source
Fantendo. http://fantendo.wikia.com/wiki/Fantendo:Main_Menu (accessed: April 12, 2013).
Fantendo. http://fantendo.wikia.com/wiki/Fantendo:Main_Menu (accessed: April 12, 2013).