Evans Aldol Reaction: Mechanism
An Evans aldol reaction takes place between substrate 6 and 19 to generate the desired isomer, 20
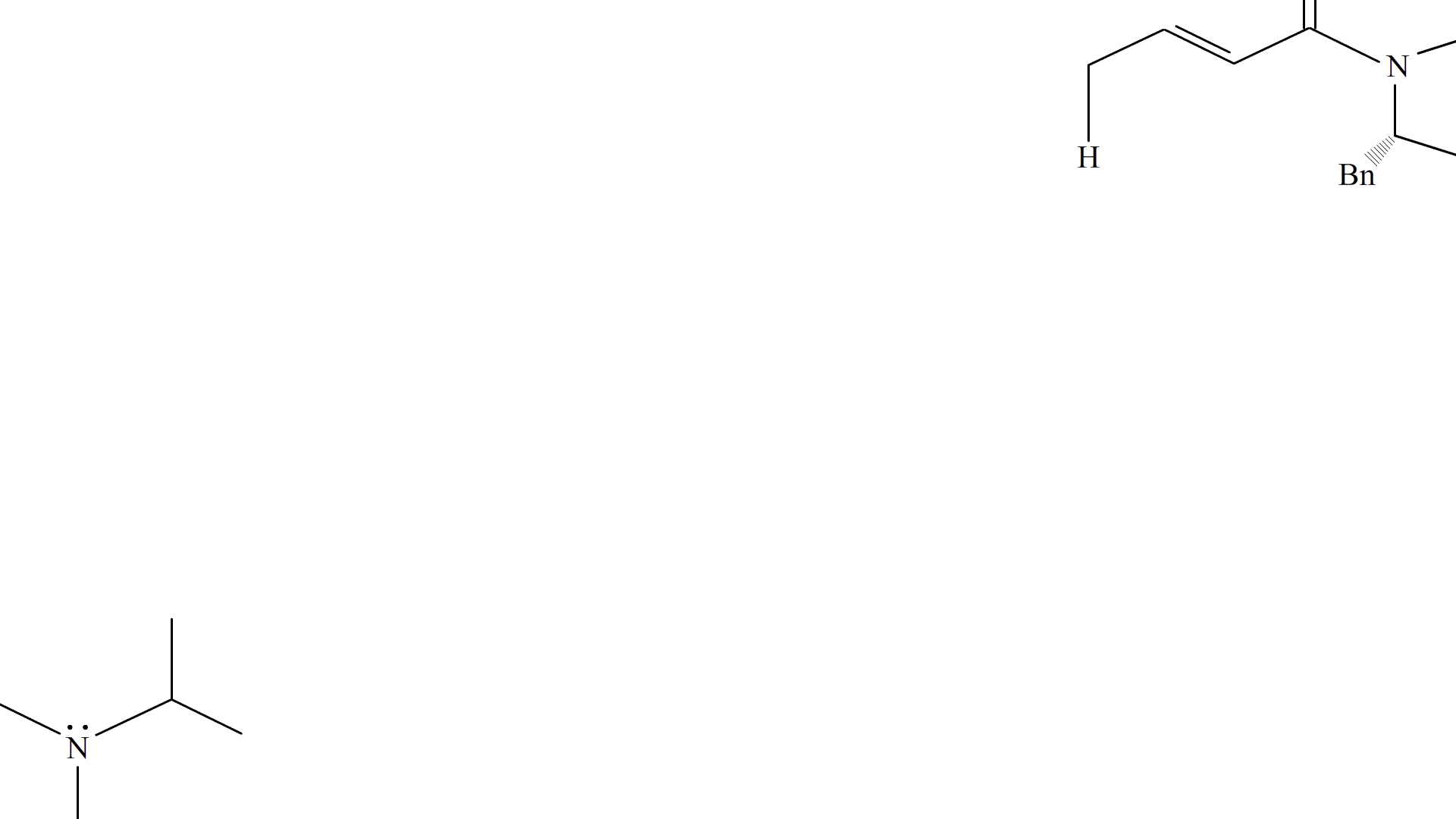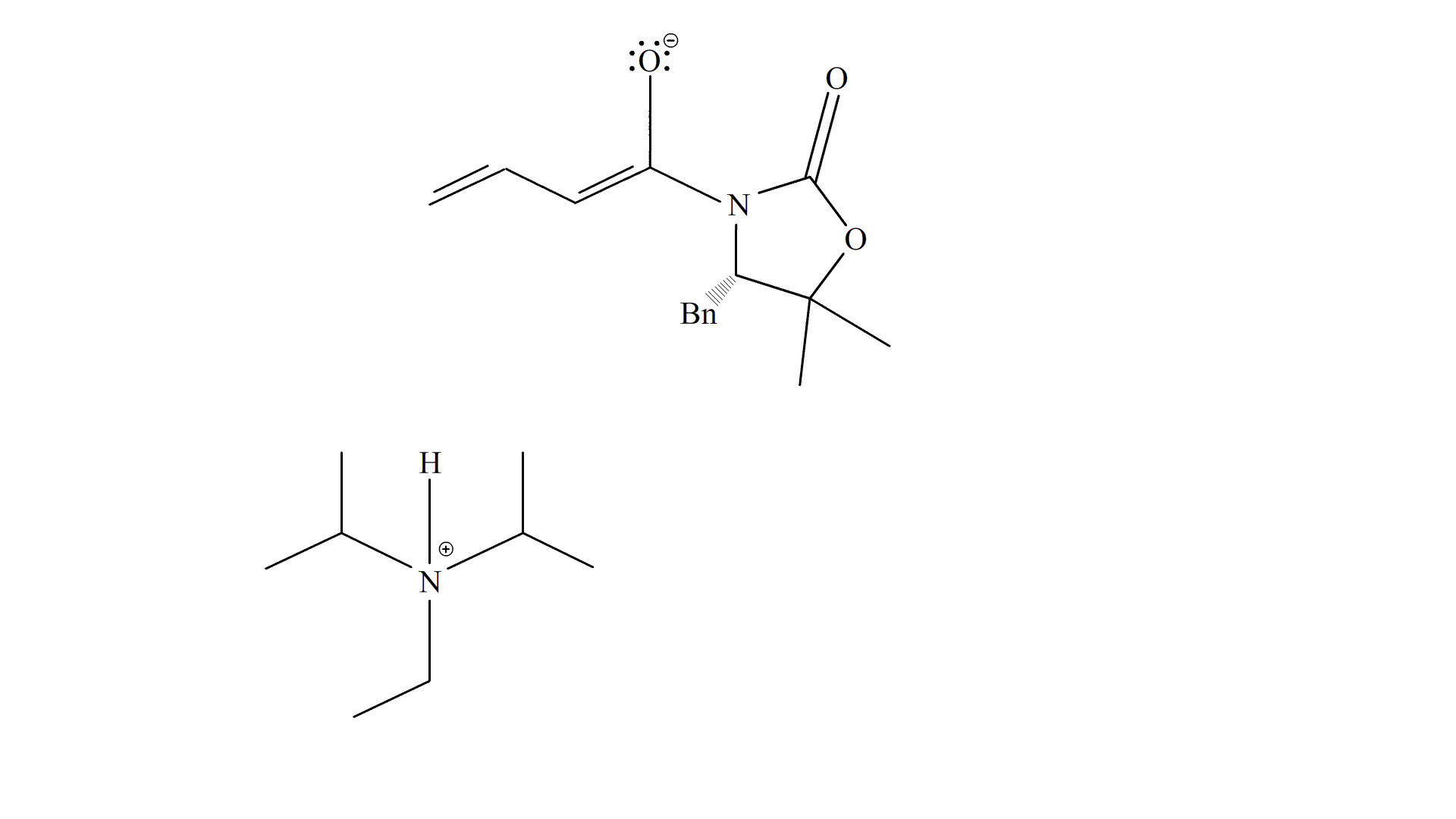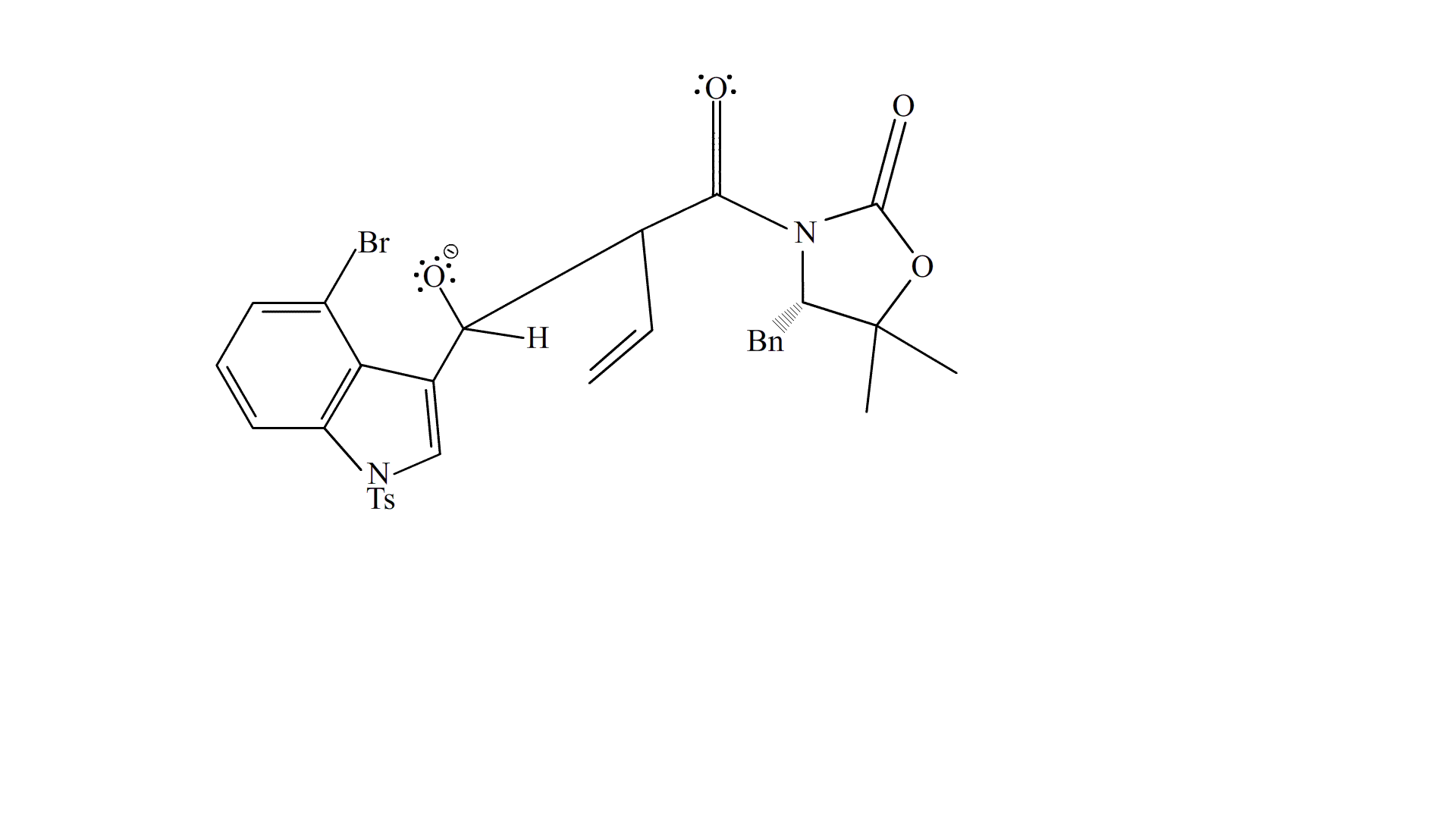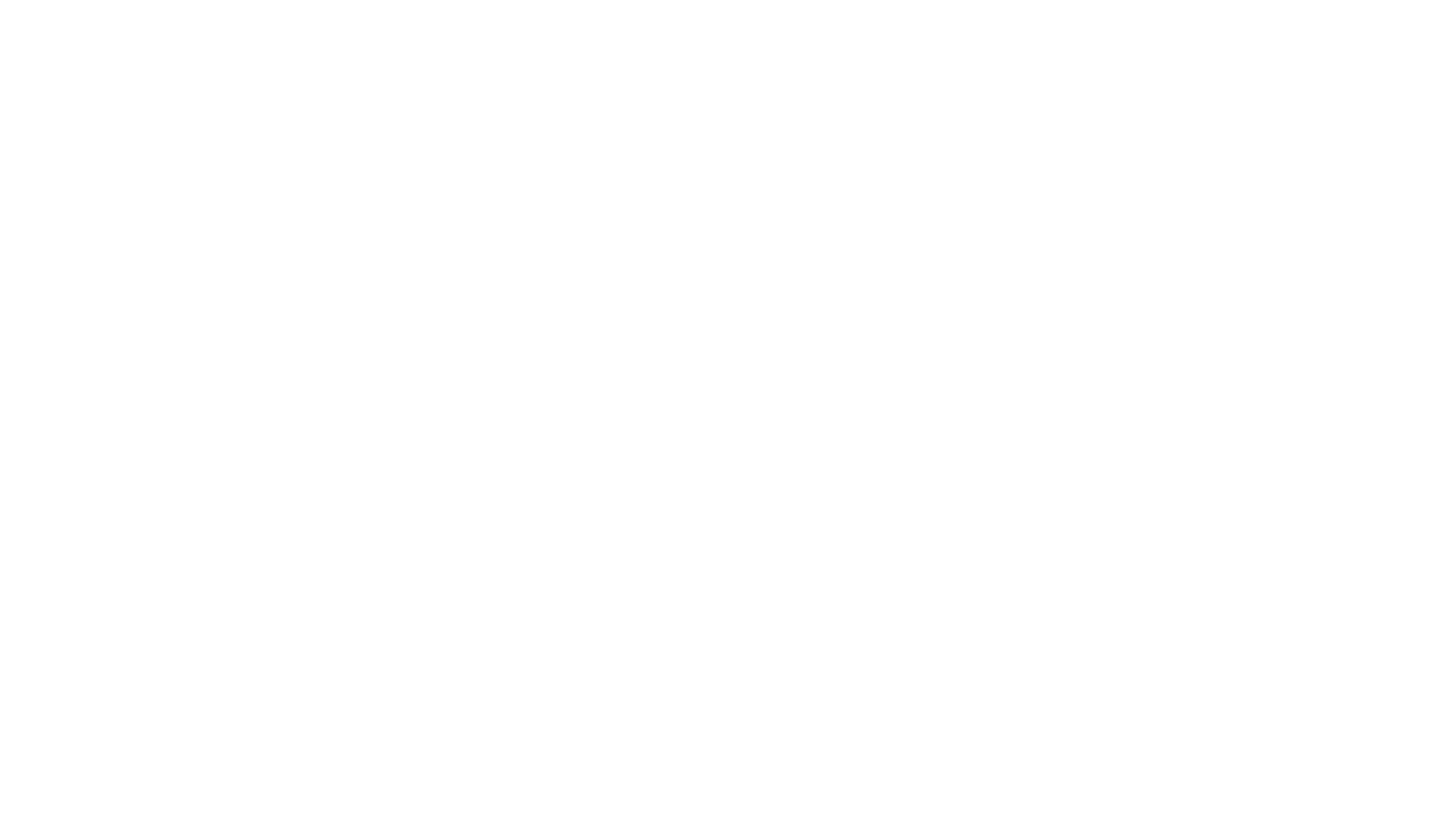
i-Pr2NEt deprotonates the methyl group at the end of Substrate 6, creating an alkene at the carbon and its adjacent carbon, and resonating the adjacent alkene to form an enol at the carbonyl. The carbonyl reforms and the enol breaks down to attack the carbonyl on Substrate 19, forming an oxygen anion which gets protonated (most likely by i-Pr2NEt) to form the product, Substrate 20.
