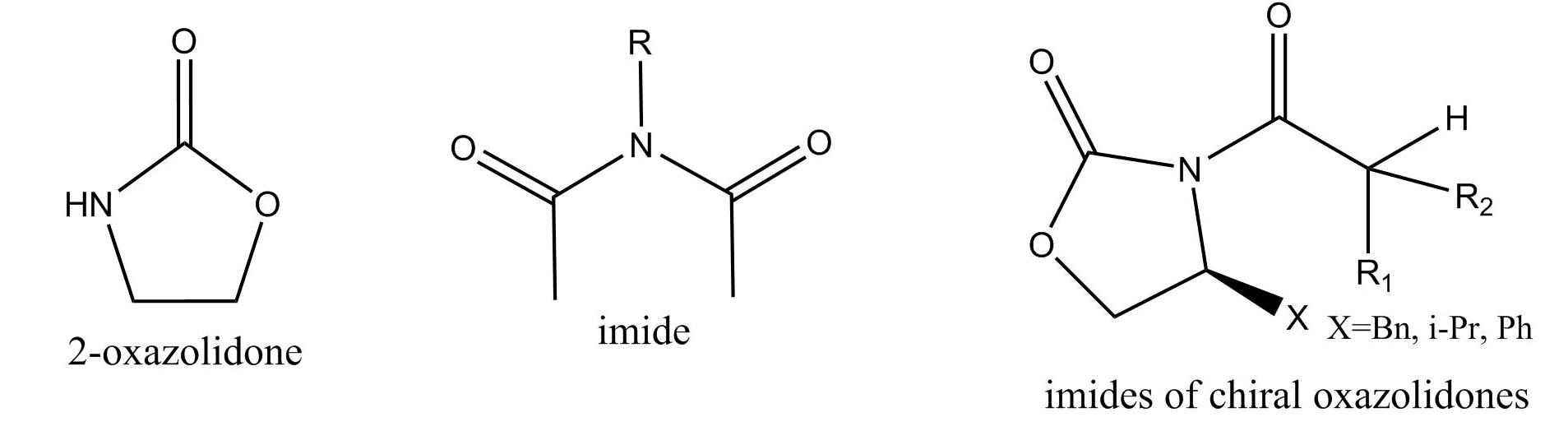
Evans Aldol Reaction: Tutorial
The Evans Aldol reaction takes place specifcally on imides of chiral oxazolidones. An imide is a function group consisting a nitrogen placed in between two carboxyl groups. An oxazolidone is deriviative of the molecule 2-Oxazolidone.
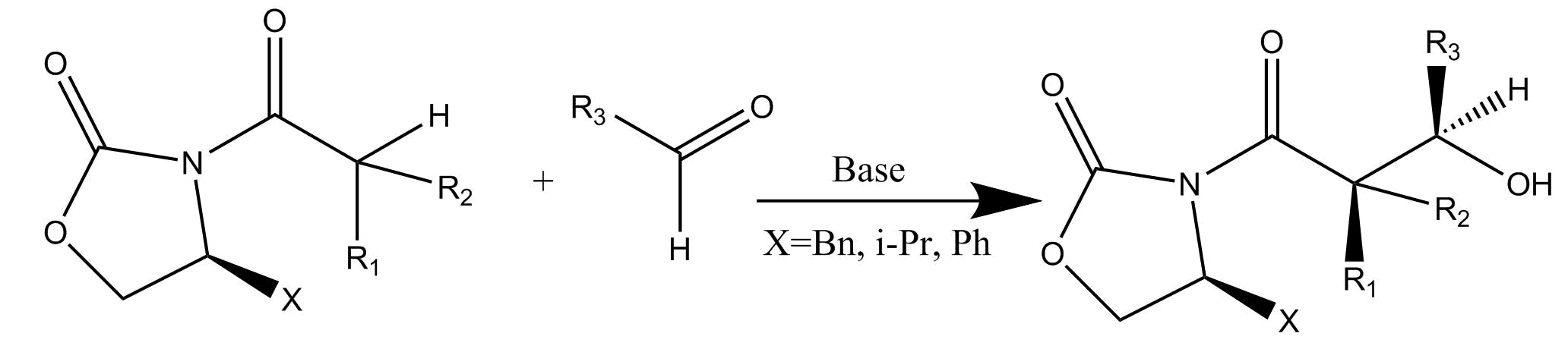
In the Evans Aldol reaction, imides of chiral oxazolidones are reacted with an aldehyde in the presence of base.
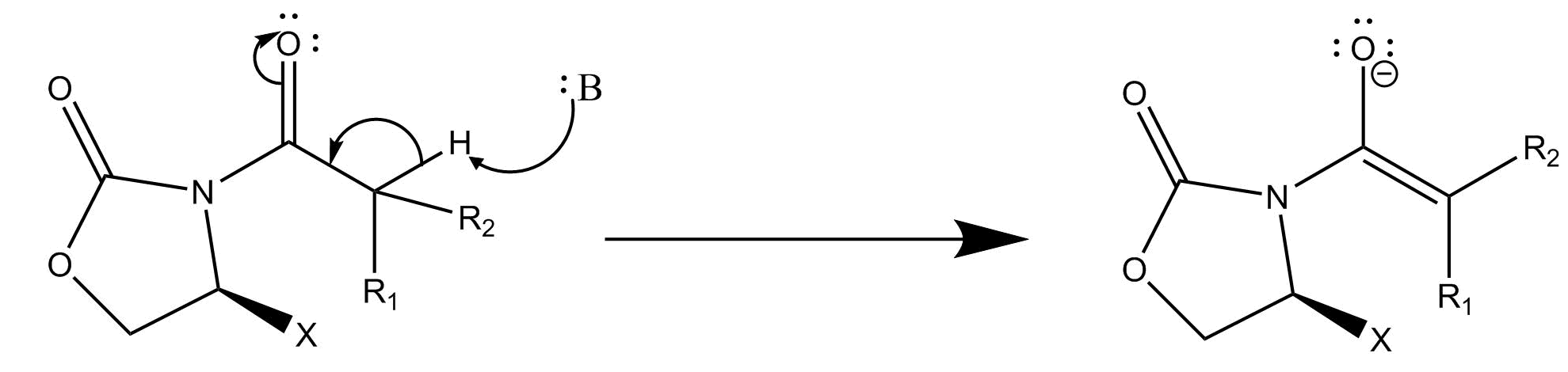
The first step of the reaction consists of deprotnating to form an enolate
Enolates are nucleophiles and aldehyde carbons are electrophiles, so the enolate attacks the aldehyde
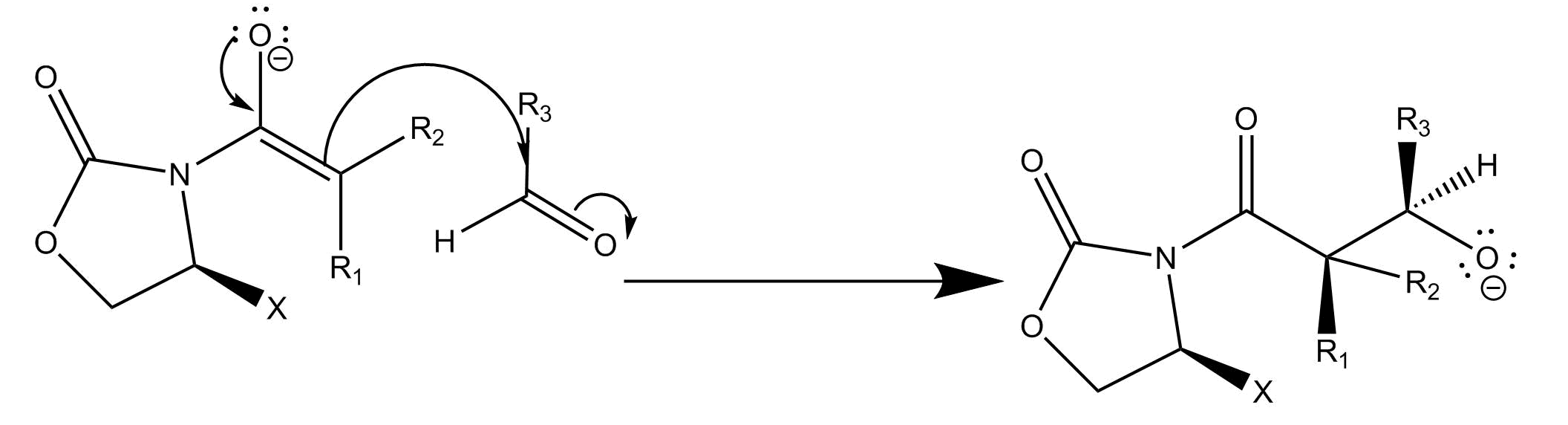
Finally, deprotonation occurs:
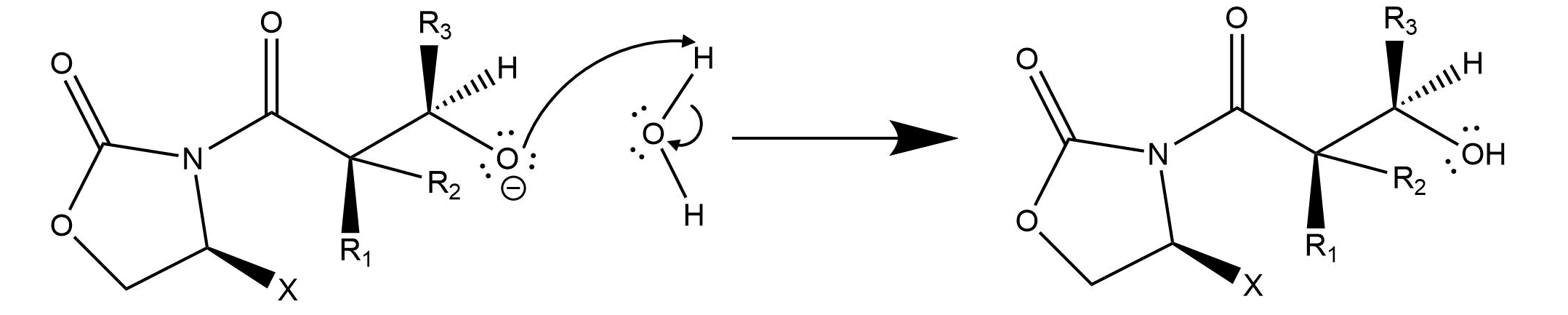
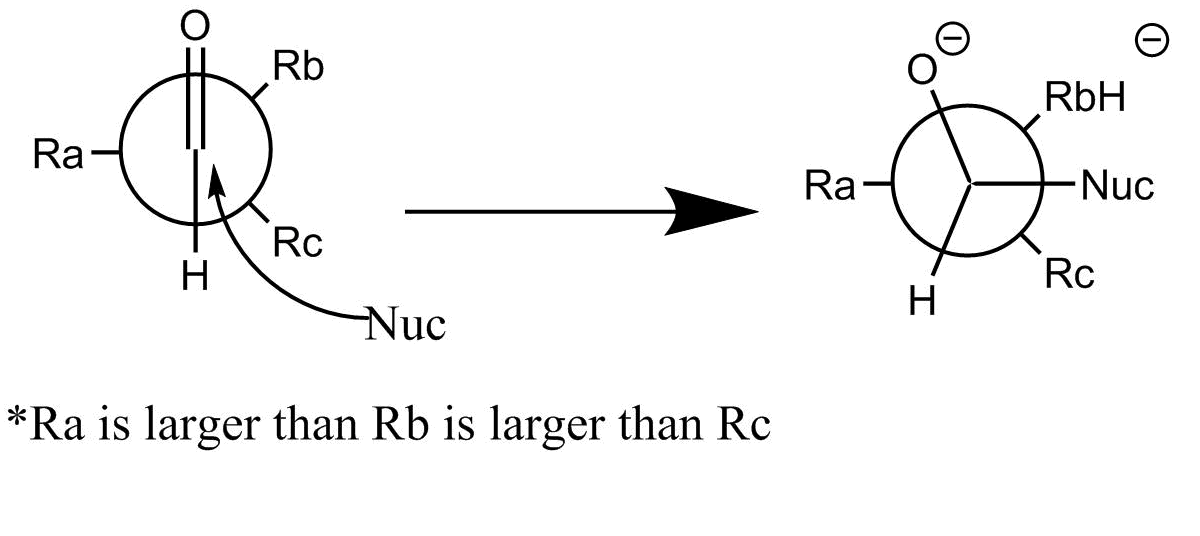
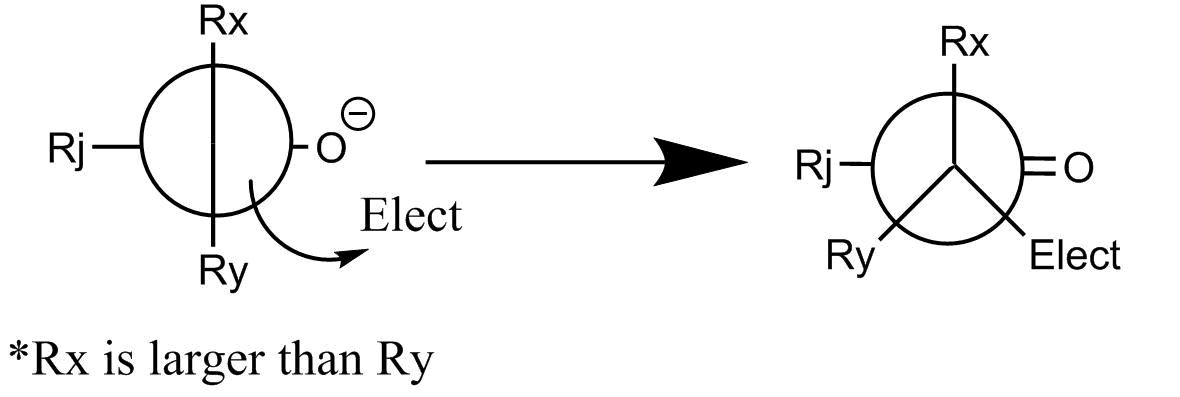
What is interesting about this reaction is its high stereoselectivity. This results from the second, in which the enolate attacks the carbonyl group. If we look at the Newman projection of the functional group in each molecule, the reaction occurs at the least sterically hindered angle. This produces the stereochemistry of the resulting product.
|
|