Evans Aldol Reaction: References
Evans, D. A.; Sjogren, E. B.; Bartroli, J.; Dow, R. L. Tetrahedron Lett. 1986, 27 (41), 4957-4960.
Three References to the paper by Dr. David A. Evans from Tetrahedron Letters cited above:
Wang, H.; Matsuhashi, H.; Doan, B. D.; Goodman, S. N.; Ouyang, X.; Clark, W. M. Tetrahedron 2009, 65 (32), 6291-6303.
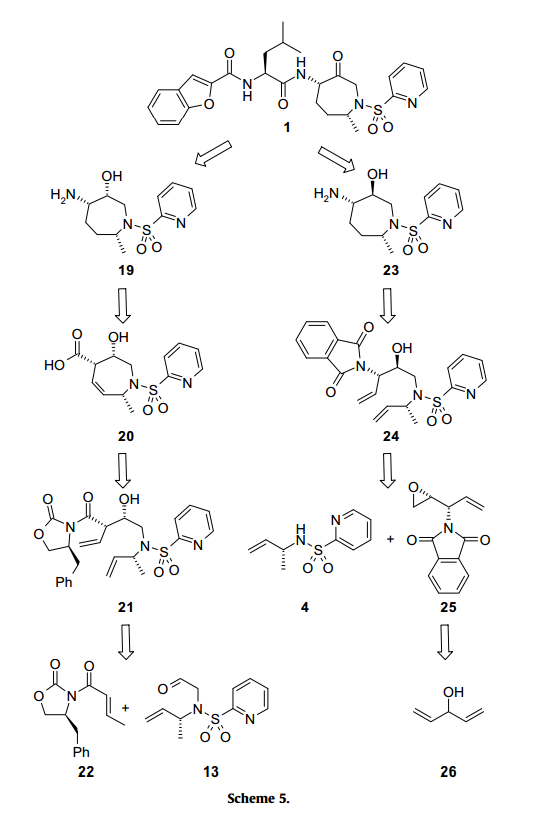
In the synthesis of SB-462795, a Cathespin K inhibitor (Cathespin K is an enzyme found in the genes of a large fraction of humans with breast cancer), the Evans aldol condensation of an aldehyde and an imide is required to form the syn product the researchers were looking for.
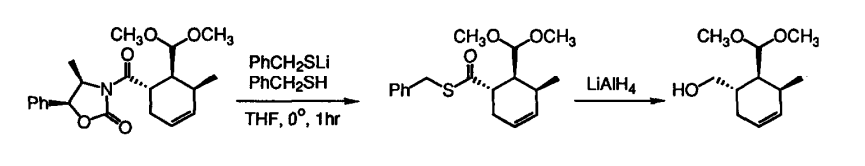
Chiral Evans-type oxazolidones are used in many reactions (such as our reaction) to induce asymmetry, however, sometimes they can be hard to remove. This paper discusses how to remove them using benzyl mercaptan to get the thioester they wanted.
Fürstner, A. Chem. Rev. 1999, 99 (4), 991-1046.
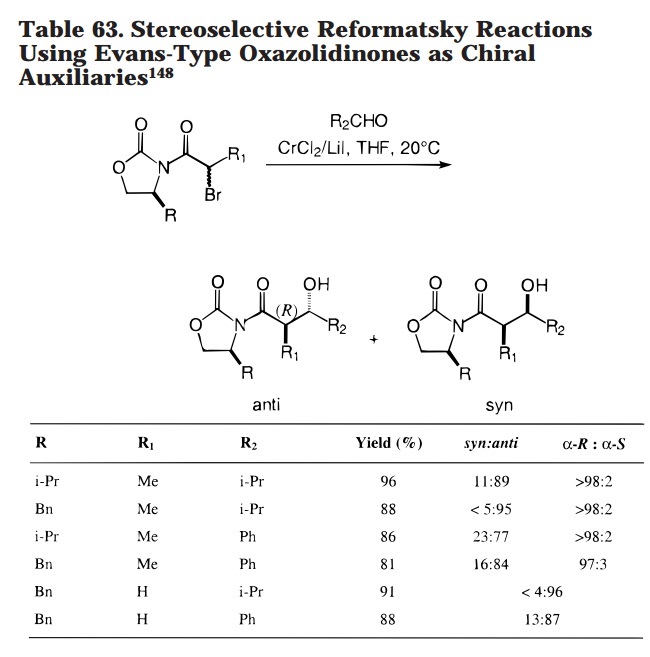
An Evans-type oxazolidones was used in a Reformatsky reaction (not our reaction) to get high selectivity as well.