1H-NMR
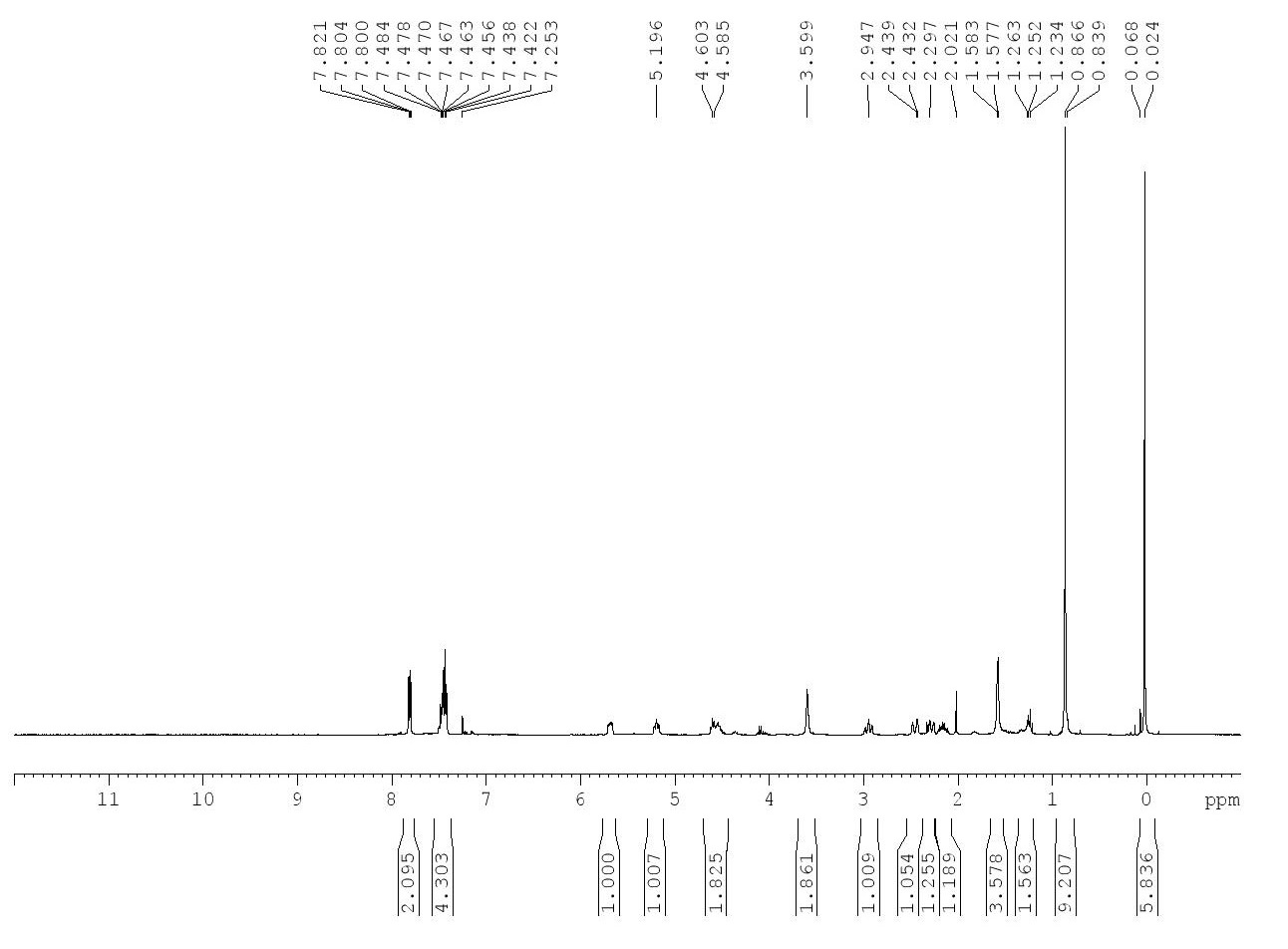
| Chemical Shift (in ppm) | Integration | Multiplicity | Justification |
|---|---|---|---|
| 7.82-7.80 | 2 H | d | Aromatic hydrogens closer to EWG |
| 7.48-7.42 | 4 H | m | Aromatic hydrogens further from EWG |
| 5.72-5.66 | 1 H | m | Alkene hydrogen further from Nitrogen EDG |
| 5.52-5.19 | 1 H | t | Alkene hydrogen closer to Nitrogen EDG |
| 4.63-4.50 | 2 H | m | Pair of hydrogens on a carbon next to an electron-withdrawing oxygen shifts the signal downfield |
| 3.60 | 2 H | s | The amine hydrogen and electron-dense alkene hydrogen in close proximity produce a signal at 3.60 with an integration of 2 |
| 2.99-2.91 | 1 H | t | Alkyl hydrogen in presence of electron-withdrawing elements in compound structure |
| 2.48-2.44 | 1 H | d | Alkyl hydrogen in presence of electron-withdrawing elements in compound structure |
| 2.31-2.24 | 1 H | d | Alkyl hydrogen in presence of electron-withdrawing elements in compound structure; slightly further to EWG |
| 2.20-2.10 | 1 H | m | Alkyl hydrogen in presence of electron-withdrawing elements in compound structure; slightly further to EWG |
| 1.58-1.57 | 4 H | m | Integration of 4 and chemical shift of around 1.5 indicates high probability of alkane C-H |
| 1.26-1.23 | 1 H | m | R3CH with presence of electron-donating elements in compound structure |
| 0.86 | 9 H | s | Integration of 9 and singlet indicates tert-butyl group |
| 0.02 | 6 H | s | Integration of 6 and singlet indicates two methyl groups attached to an atom with no hydrogens |
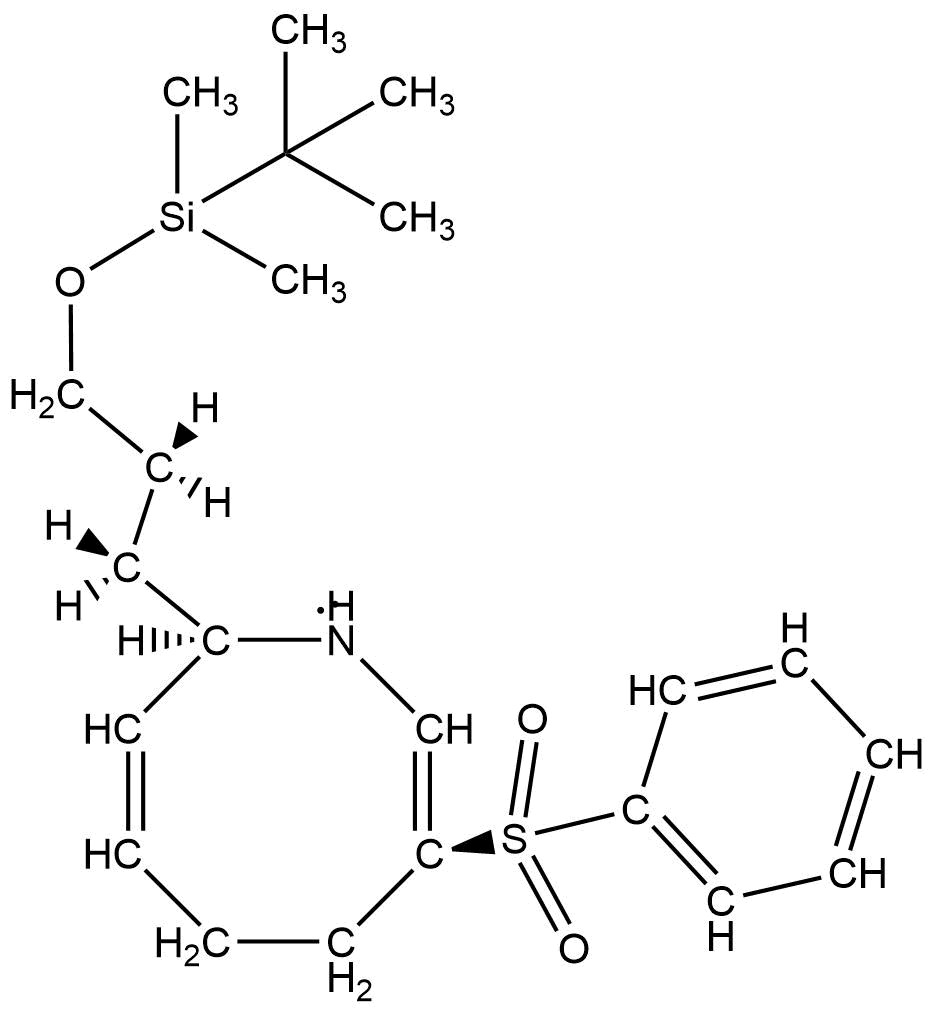
The molecules analyzed were part of the mechanism of the synthesis of (−)-nakadomarin A. (−)-Nakadomarin A is a biologically active compound believed to have inhibitive, antitumor properties. (−)-Nakadomarin A is highlighted in the scientific community for its unique, complex structure. The authors pursued a concise synthesis of this medicinally important molecule from achiral or racemic starting materials. Molecules 14 and 15 are intermediates in the synthesis of (−)-Nakadomarin A.
No 1H NMR spectrum was provided for compound 14.

For compound 15, the protons were assigned according to the chemical shift values, integrations, and multiplicities provided by the 1H NMR data. The presence of atoms like oxygen and nitrogen, as well as considerations of R group substituents were taken into account while assigning the signals for the 1H NMR data provided in the supplemental information.
Some issues were encountered while analyzing the 1H NMR data provided in the supplemental information. For instance, looking at the structure of compound 15, there are no true hydrogen atoms which would result in a singlet with an integration of 2, with an expected chemical shift of 3.6. This likely resulted from a contamination of the product or the solvent in the NMR tube. Additionally, the signal (b) indicated that the integration was 4H, but again, this does not correlate with the structure of compound 15.