References
Main Paper: A Scalable Total Synthesis of (-)-Nakadomarin A.
Citation: Boeckman, R. K., Jr.; Wang, H.; Rugg, K. W.; Genung, N. E.; Chen, K.; Ryder, T. R. Org. Lett. 2016, 18, 6136-6139.
Reference: (33) Boeckman, R. K., Jr.; Flann, C. J.; Poss, K. M. J. Am. Chem. Soc. 1985, 107, 4359.
Description: Boeckman et al. used a previous synthesis developed in their lab as a reference for their rationale in their conversion of compound 14 to compound 15 via the retro-aza-Claisen rearrangement in the main paper, listed above.

The following papers also cited Boeckman’s paper (1985) in explaining their various syntheses, employing similar chemistry.
Paper 1: Enantioselective Total Synthesis of (+)-Salvileucalin B.
Citation: Levin, S.; Nani, R. R.; Reisman, S. E. J. Am. Chem. Soc. 2011, 133, 774-776.
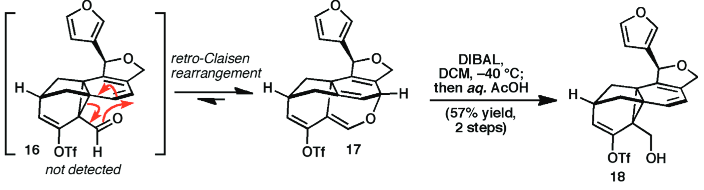
Figure 1: Levin, S.; Nani, R. R.; Reisman, S. E. J. Am. Chem. Soc. 2011, 133, 775.
Description: This article refers to the reversible ring-opening Claisen rearrangements studied in the referenced article. Levin et. al comment on the difficulties they experienced in synthesizing their desired compounds, because their desired aldehyde arrangement was less favorable than the Claisen rearrangement. However, they were nonetheless able to use an appropriate reducing agent to obtain the primary alcohol they required for their synthesis.
Paper 2: Nimbolide and isonimbolide.
Citation: Anitha, G.; Raj, J. J. L.; Narasimhan, S.; Solomon, K. A.; Rajan, S. S. J. Asian. Nat. Prod. Res. 2006, 8, 445-449.
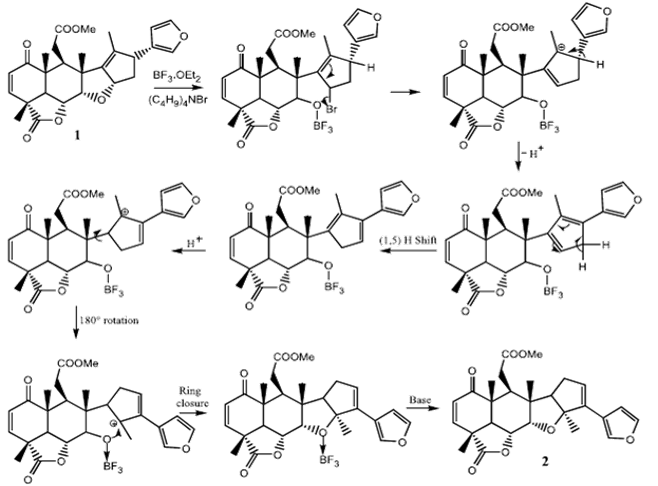
Figure 2: Anitha, G.; Raj, J. J. L.; Narasimhan, S.; Solomon, K. A.; Rajan, S. S. J. Asian. Nat. Prod. Res. 2006, 8, 447.
Description: In this paper, the scientists attempted to isolate nimbolide, a biologically active, medicinal compound from neem leaves. They also explored the bioactivity and structure of nimbolide by cleaving an ether linkage in their compound, using several reagents. They discussed a proposed mechanism for the cleavage of the ether, and conversion to the structurally isomeric compound isonimbolide, seen in Figure 2. In order to explain the [9, 10] rearrangement of the double bond, and subsequent ring closure in their mechanism, the authors cited Boeckman’s paper and its exploration of the Claisen rearrangement.
Paper 3: Asymmetric Synthesis of (-)-Trichodiene. Generation of Vicinal Stereogenic Quaternary Centers via the Thio-Claisen Rearrangement.
Citation: Lemieux, R. M.; Meyers, A. I. J. Am. Chem. Soc. 1998, 120, 5453-5457.
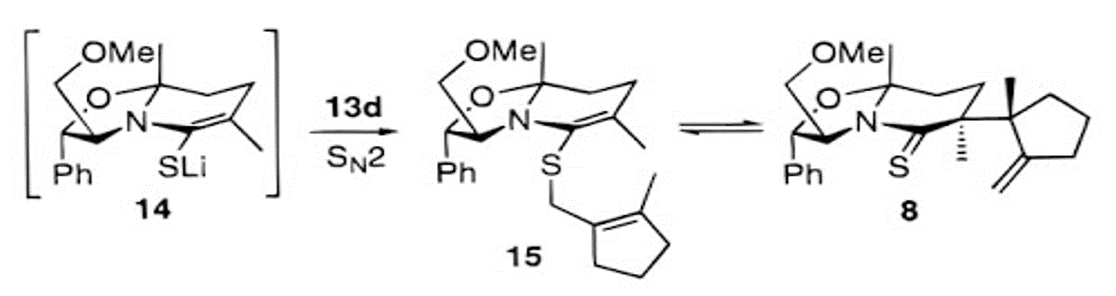
Figure 3: Lemieux, R. M.; Meyers, A. I. J. Am. Chem. Soc. 1998, 120, 5454.
Description: In this paper, the scientists noticed an equilibrium between compound 15 and compound 8. In the conversion between thioenamine 15 and thiolactam 8, the experimental commented on the low conversion rate (compounds 15 and 8 were present in a 60:40 ratio, according to their 1H-NMR). The scientists rationalized the low conversion rate, based on the knowledge of Claisen rearrangement’s reversibility, which they attributed to Boeckman’s synthesis (Boeckman, R. K., Jr.; Flann, C. J.; Poss, K. M. J. Am. Chem. Soc. 1985, 107, 4359).