Leading Question
What is the Staudinger Reaction?
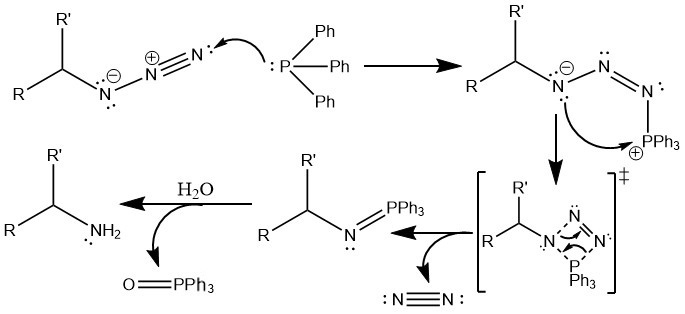
The Staudinger Reaction converts an -N3 group (azide) to a -NH2 group (amine.) For this reaction to occur, an azide, and the reagents PPh3 (triphenylphosphine) and H2O (aqueous workup) are required.
First, the PPh3 reacts with the azide to generate a phosphazide intermediate. One pair of electrons in the triple-bond in the azide is bumped onto the central nitrogen, and the phosphorus atom donates a free electron pair to the outermost nitrogen atom of the azide.
Next, the compound loses nitrogen gas. A rectangular intermediate forms, and the product is stabilized by an interaction between the innermost nitrogen atom and the triphenylphosphine component.
Finally, the treatment of this compound in water replaces the N=PPH3 compound into the NH2 compound, with the H2 coming from the water. The water molecule acts as a nucleophile toward the phosphorus atom, which pushes electrons onto the nitrogen atom, results in a cationic oxygen and anionic nitrogen intermediate. The oxygen gets deprotonated by water, and then the H3O+ protonates the anionic nitrogen. The HO-PPH3 component is stable enough to leave, leaving a negatively charged nitrogen again. This deprotonates the HO-PPH3 component. This final reduction step results in the formation of an amine, and a very stable, uncharged phosphine oxide compound.