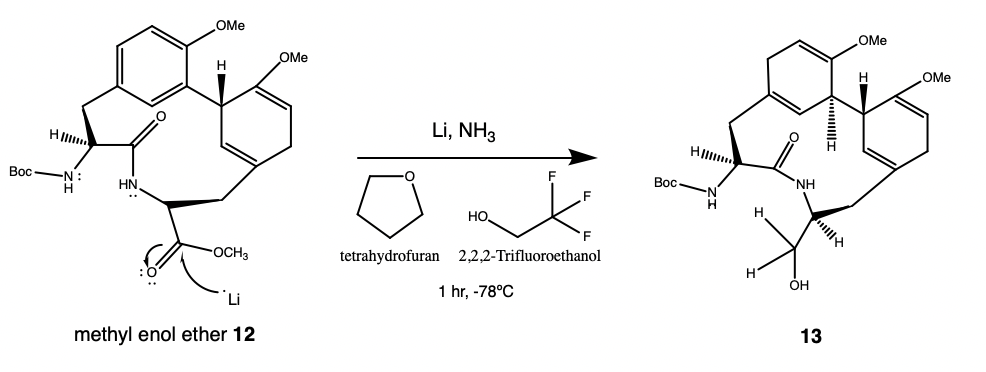
Experimental Procedure to Create Product 13
10 ml of anhydrous ammonia (NH3) was condensed at -78 degrees Celsius in a flask connected to an Argon filled balloon. 53 mg of the lithium (Li) metal was dissolved in NH3 with 5 minutes of rapid stirring.
A solution containing 100 mg of methyl enol ether 12 in 2.1 ml of the tetrahydrofuran/2,2,2-trifluoroethanol (THF/CF3CH2OH) was added to the Li/NH3 reagent dropwise over 7 minutes. The reaction mixture was stirred for one hour, and then 500 mg of solid NH4Cl was added portion-wise until the NH3 solution appeared colorless.
The NH3 was evaporated for over one hour by removal of cooling bath. The remaining white residue was diluted with 20 ml of water and 20 ml of brine. The aqueous solution was extracted with 25 ml of dichloromethane (DCM) five times and dried over Na2SO4 and concentrated under vacuum to give 113 mg of crude 13.

3 Comments
SJ Song
4 weeks agoReply*Rolls in 30 minutes late* Hey guys guess how much I slept in the last week
Akira Nishii
5 weeks agoReplyOh god not this again