Understanding the mechanism to making the purest meth is the key to making money. Let's get this bread.
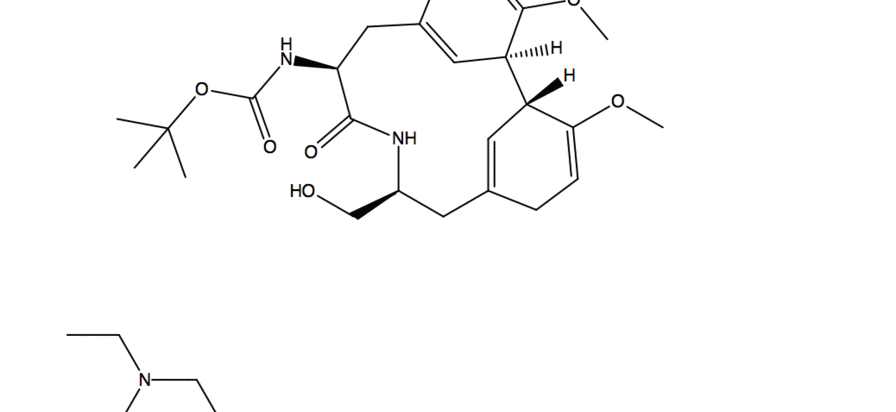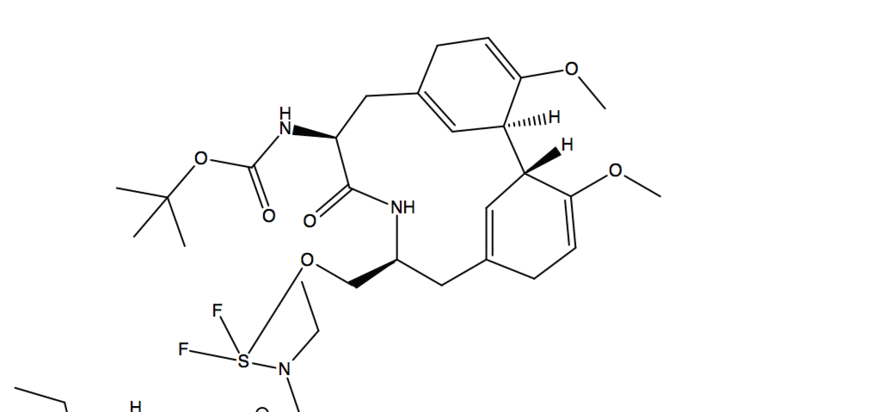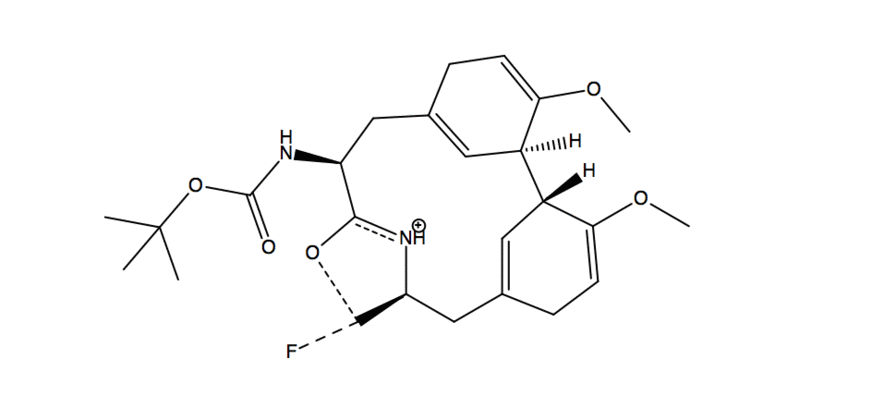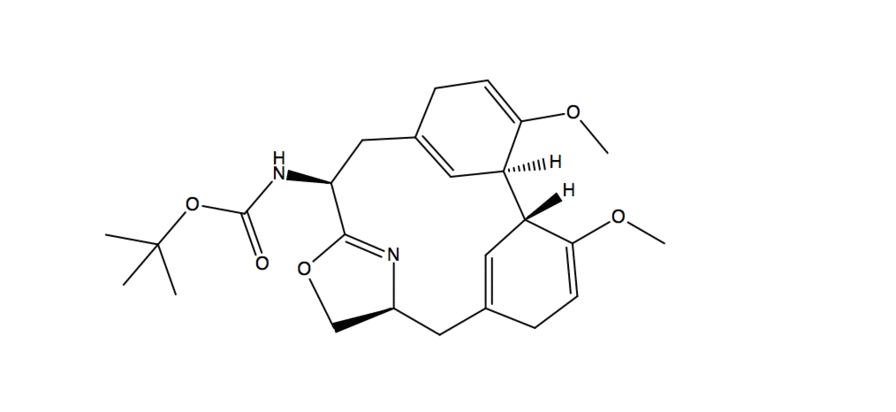
- Substitution reaction with -OH acting as nucleophile and fluorine from DAST acting as electrophile
- A deprotonation occurs with another molecule of DAST
- Fluorine than acts as a nucleophile and in a substitution reaction it kicks off the O-DAST complex
- In a ring closing reaction similar to an assisted ionization reaction, the flow of electrons goes from Nitrogen onto the carbonyl Oxygen which then reacts in another substitution reaction kicking of fluorine and creating a new C-O bond
- In the work up conditions the reaction was quenched under basic conditions(using sodium bicarbonate) resulting in a neutral product.

