Additional Homework Problems
- CDP10-AB Suggest a rate law and mechanism for the
catalytic oxidation of ethanol over tantalum oxide when the adsorption of ethanol and
oxygen takes place on different sites. [2nd Ed. P6-17]
- CDP10-BB Analyze the data for the vapor-phase
esterification of acetic acid over a resin catalyst at 118 C.
- CDP10-CB Silicon dioxide is grown by CVD according
to the reaction SiH2Cl2(g) + 2N20(g)
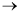 SiO2(s) +
2N2(g) + 2HCL(g). Use the rate data to determine the rate law, reaction
mechanism, and rate law parameters. [2nd Ed. P6-13]
SiO2(s) +
2N2(g) + 2HCL(g). Use the rate data to determine the rate law, reaction
mechanism, and rate law parameters. [2nd Ed. P6-13]
- CDP10-DB Determine the rate law and rate law
parameters for the wet etching of an aluminum silicate.
- CDP10-EB Titanium films are used in decorative
coatings as well as wear-resistant tools because of their thermal stability and low
electrical resistivity. TiN is produced by CVD from a mixture of TiCl4 and NH3TiN.
Develop a rate law, a mechanism, and a rate-limiting step, and evaluate the rate law
parameters.
- CDP10-FB The dehydrogenation of ethylbenzene is
carried out over a Shell catalyst. From the data provided, find the cost of the catalyst
required to produce a specified amount of styrene. [2nd Ed. P6-20]
- CDP10-GB The formation of CH4 from CO
and H2 is studied in a differential reactor.
- CDP10-HA Determine the rate law and mechanism for
the reaction A + B
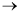 C.
C.
- CDP10-IB Determine the rate law from data where
the pressures are varied in such a way that the rate is constant. [2nd Ed. P6-18]
- CDP10-JB Determine the rate law and mechanism for
the vapor phase dehydration of ethanol. [2nd Ed. P6-21]
- CDP19-KB Analyze the rate data
- CDP10-LB Carbonation of allyl chloride whereby the complexes Pd*CO and Pd*CO*NaOH* are formed. Find the rate limiting step. [3rd Ed. P10-11]
Catalyst Decay
- CDP10-MB California problem. Isomerization reaction with catalyst decay. Review the data and make a recommendation. [3rd Ed. P10-18]
- CDP10-NB Fluidized bed reactor with catalyst decay as measured by decline in octane number. Real data. [3rd Ed.P10-20]
- CDP10-OB Catalyst decay in a catch reactor. [3rd Ed. P10-23]
- CDP10-PB Deactivation by coking in a differential reactor. [3rd Ed. P10-24]
- CDP10-QB The autocatalytic reaction A + B
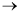 2B is carried out in a
moving-bed reactor. The decay law is first-order in B. Plot the activity and the
concentrations of A and B as a function of catalyst weight.
2B is carried out in a
moving-bed reactor. The decay law is first-order in B. Plot the activity and the
concentrations of A and B as a function of catalyst weight.
- CDP10-RC The decomposition of cumene is carried out over a LaY zeolite catalyst, and deactivation is found to occur by coking. Determine the decay law and rate law, and use these to design a STTR. [2nd Ed. P6-27]
- CDP10-SB A second-order reaction over a decaying catalyst takes place in a moving-bed reactor. [Final Exam, Winter 1994]
- CDP10-TB A first-order reaction A
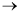 B + C takes place in a moving-bed reactor. [Final Exam, Winter 1994]
B + C takes place in a moving-bed reactor. [Final Exam, Winter 1994]
- CDP10-UB For the cracking of normal paraffins (Pn), the rate has been found to increase with increasing temperature up to a carbon number of 15 (i.e., n < 16) and to decrease with increasing temperature for a carbon number greater than 16. [J. Wei, Chem. Eng. Sci., 51, 2995 (1996)]
- CDP10-VB The reaction A + B
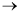 C + D is carried out in a moving-bed reactor.
C + D is carried out in a moving-bed reactor.
- CDP10-WB Second order reaction and zero order decay in a batch reactor.
- CDP10-XB First order decay in a moving bed reactor for the series reaction A
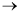 B
B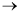 C
C