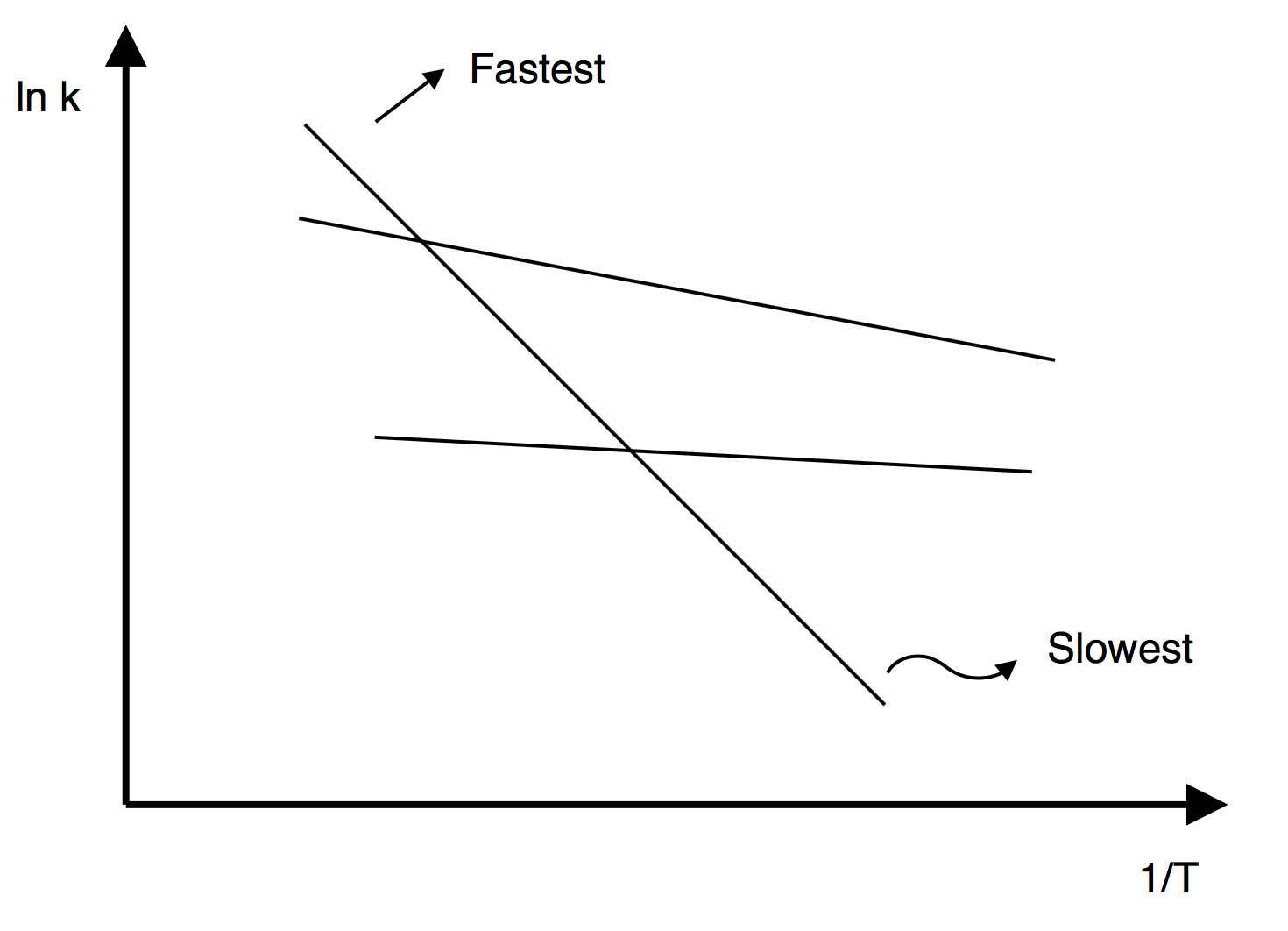
Chapter 3: Rate Laws
Activation Energies
Consider the following elementary reactions
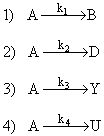
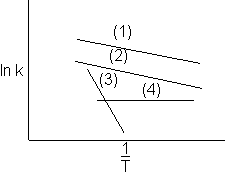
1) Which reaction has the higher activation energy?
2) Which reactions have the same activation energy?
3) Which reaction is virtually temperature insensitive?
4) Which reaction will dominate (i.e. take place the fastest) at high temperatures if all reactions were to take place in the same reactor?
![]()
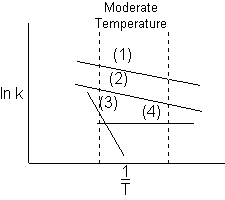
5) Which reaction will dominate (take place the fastest) at moderate temperatures?
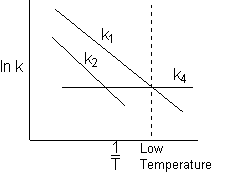
6) Which reaction will take place the fastest at low temperatures?
![]()
7) Which reactions will have the same rate of reaction at a given temperature?
8) Which reactions will dominate at high temperatures and be the slowest at low temperatures?
Solutions
Consider the following elementary reactions

1) Which reaction has the higher activation energy?

Ans. (3)
2) Which reactions have the same activation energy?
Ans. (1) and (2) – Slope (–E/R) is the same.
3) Which reaction is virtually temperature insensitive?
Ans. (4). k4 does not vary with temperature E4 = 0.
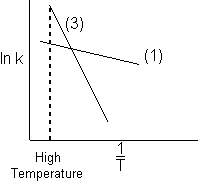
4) Which reaction will dominate (i.e. take place the fastest) at high temperatures?

![]()
At high temperatures (1/T) is small and k3 becomes larger than k1. ![]()
Ans. (3)
5) Which reaction will dominate (take place the fastest) at moderate temperatures?

At moderate temperatures ![]()
Ans. (1)
6) Which reaction will take place the fastest at low temperatures?

![]()
At low temperature (1/T) is larger. ![]()
Ans. (4)
7) Which reactions will have the same rate of reaction at a given temperature?
Ans. The rate will be the same when the lines cross.
a. (1) and (3)
b. (2) and (3)
c. (1) and (4)
d. (2) and (4)
e. (3) and (4)
The activation energy is a measure of the minimum energy a that the reacting molecules must have in order for the reaction to occur.
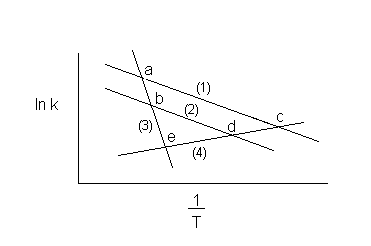
7) Which reactions will dominate at high temperatures and be the slowest at low temperatures?
Ans. Reaction (1)