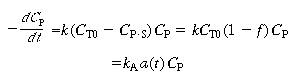Chapter 10: Catalysis and Catalytic Reactors
Learning Resources
Example CD10-4: Catalyst Poisoning in a Constant-Volume Batch Reactor
Equation numbers refer to DVD Chapter 10
Develop a decay Equation for catalyst poisoning solely as a function of catalyst activity.
Solution
For the case of a constant-volume batch reactor, a mole balance of the poison in the gas phase combined with the rate law for poisoning [Equation (10-109)] gives |
|||
|
|
(CD10-4.1) |
||
| where k A =kC T0 is the initial total concentration of unpoisoned site, C P•S is the concentration of the poisoned sites, and ƒ is the fraction of the poisoned sites. Taking the ratio of Equation (10-111) to Equation (CD10-4.1) |
|||
| and then integrating with limits a = 1 and C P =C P0 |
|||
| (CD10-4.2) |
|||
| or |
|||
(CD10-4.3) |
|||
|
Constant- |
The decay can now be written in the form |
||
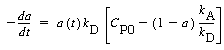
|
(CD10-4.4) |
||
|
which is of the form: |
|||
(CD10-4.5) |
|||
where |
|||
Back Next |