
Chemistry molecules are as beautiful as a Krabby Patty! Checkout the
proton assignments for molecule 3 below.
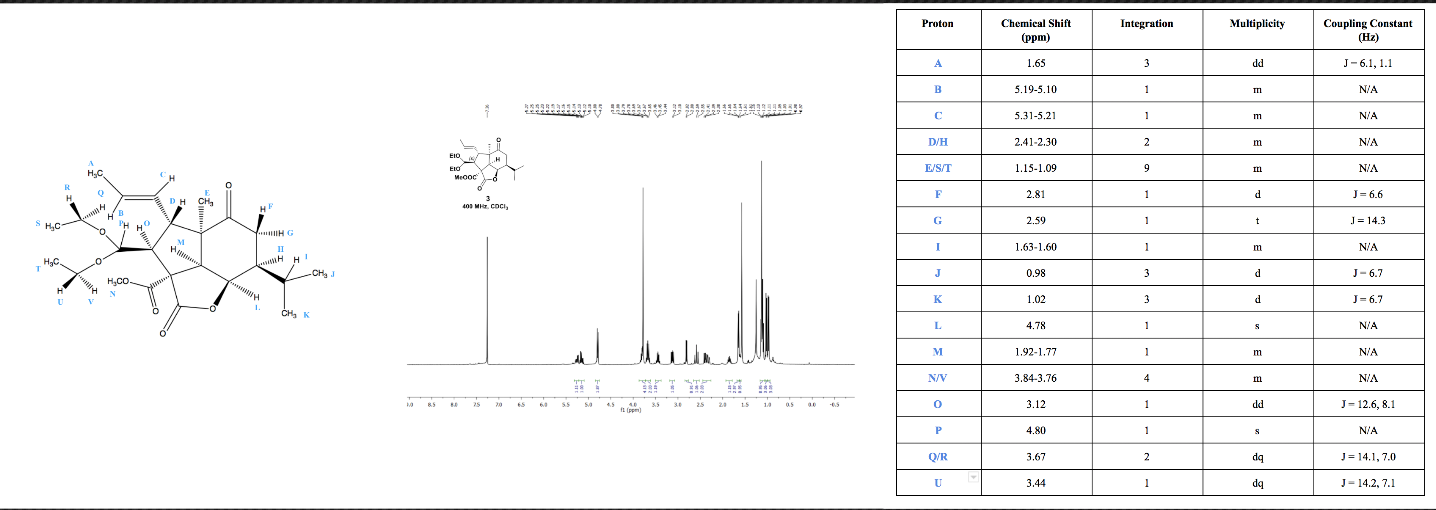
A: Group A protons should show up as a doublet of doublets due to the differing J values on each alkene proton. Furthermore, the peak assigned has an integration of 3H, matching with the 3 protons of this specific methyl group.
B and C: Alkene protons typically show up around 5.3 ppm. Proton C is closer to the actual molecule, which has more electronegative groups than does the terminal methyl group. We expect proton C to be slighter more downfield compared to proton B.
D/H: Considering the provided COSY, protons D and H can be attributed to the multiplet between 2.41 and 2.30. Although these protons are not diastereotopic and are located far from one another, their chemical shifts just so happen to overlap.
E/S/T: the peak that integrates to 9H is likely three methyl groups that have low interactions with electronegative atoms. These three groups are not directly next to an electronegative oxygen atom, so we grouped them together as the 9H peak.
F/G: Protons F and G are diastereotopic so have different chemical shifts. Chemical shifts at 2.81 and 2.59 are expected for protons one bond away from carbonyl carbon-oxygen double bond.
I: This assignment is based on the COSY, which shows 1H interacting with protons ~1 ppm. These protons are the ones on the diastereotopic methyl groups, and they are the only interactions present besides themselves.
J/K: These two methyl groups differ slightly in their ppm due to the diastereotopicity of the isopropyl group that they are attached to. At any given time, the sigma bond of the ring to the isopropyl group will rotate, giving rise to slightly different magnetic readings from the two different methyl groups J and K.
L: This proton is fairly upfield due to it being adjacent to an ester. We would have predicted a doublet of doublet due to two non-equivalent 3-bond neighbors, but the empirical data shows an unexpected singlet.
M: Proton M is attached to an sp3 carbon with only one 3-bond hydrogen neighbor. Process of elimination suggests that this group is at 1.92 to 1.77 even though the multiplicity is a multiplet while a doublet would be the predicted multiplicity.
N/V: The methoxy hydrogens usually have a peak around ~3.8 ppm which is the case here. The integration of 4 can be explained by proton V having a similar chemical shift as the methoxy hydrogens.
O: Proton D shows coupling with a proton with chemical shift at 3.12 which can be attributed to proton O which makes sense given the doublet of doublets multiplicity. This multiplicity can be attributed to coupling with protons D and P.
P: This proton is two bonds away from an electronegative oxygen atom, making it fairly downfield. We expect a doublet from proton O, but the data shows an unexpected singlet.
Q/R: Being diastereotopic hydrogens next to a methyl group, these protons are expected to have a doublet of quartets splitting pattern. The integration also matches with 2H.
U: Proton U is similar to Q and R because of the unique doublet of quartets splitting pattern that arises from it being vicinal to a diastereotopic hydrogen (V) and the adjacent methyl group (T).

