Main Reference:
Enantioselective Total Synthesis of (-)-Pavidolide B
Zhang, P.; Yan, Z.; Li, Y.; Gong, J.; Yang, Z. J. Am. Chem. Soc. 2017, 139, 1389-12992.
Selected Reference:
Miura, K.; Fugami, K.; Oshima, K.; Utimoto, K. Tetrahedron Lett. 1988, 29, 1543-1546.
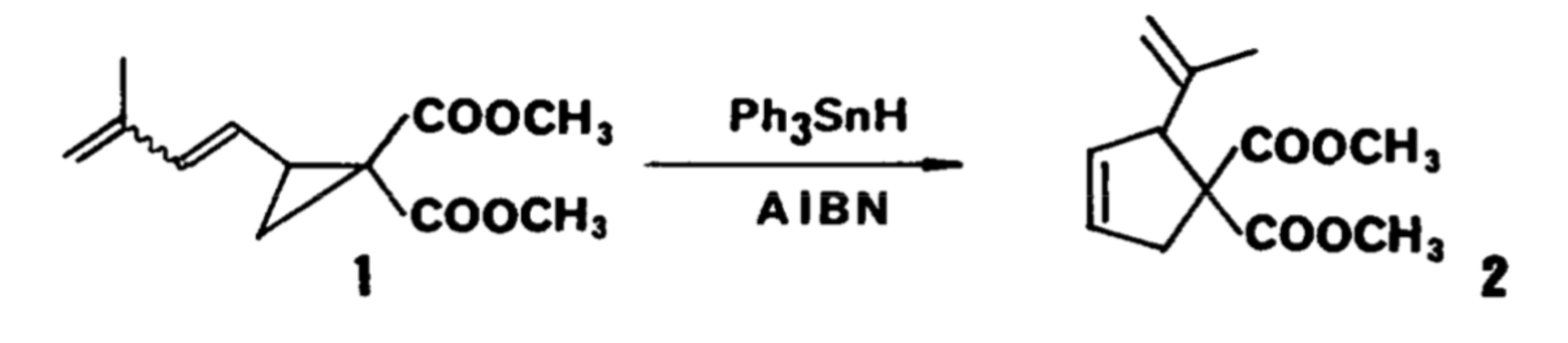
The main focus of this paper is with free radical reactions in the synthesis of organic molecules specifically in regards to transforming three-membered carbon rings to five-membered carbon rings. First, a radical source must be generated through combing a compound such as triphenyltin hydride with AIBN. When the radical source is reacted with a substrate including a cyclopropane, adjacent double bond, and a double bond somewhere else in the molecule, the radical source adds to the adjacent double bond, effectively breaking the double bond and forming a radical substrate intermediate. The radical substrate intermediate can then undergo an intramolecular reaction in which the remaining double bond and radical carbon react to ultimately form a five-membered ring. In the final step of this process, the radical intermediate undergoes loss of the initial radical source (Ph3SnH) to reform the adjacent double bond and regenerate the radical source. This paper includes the results of testing a variety of substrates with a variety of intermediates. While the percent yield of each product differs, the mechanistic pathway remains the same for all of the reactions.
Citing Papers
Vshyvenko, S.; Reed, J.; Hudlicky, T.; Piers, E. Comprehensive Organic Synthesis II 2014, 999-1076.
This paper focuses on the the diradical reaction to form a vinylcyclopentene from a vinylcyclopropane drawing specific attention to the scrambling of enantiospecificity (and sometimes stereospecificity) found in the products.
Wang, S.C.; Tantillo, D. J. J. Organomet. Chem. 2006, 691, 4386-4392.
The authors cited the experiment in this paper because there is an example of a vinylcyclopropane molecule undergoing a ring opening and closing mechanism to form a cyclopentane molecule. A diradical intermediate is formed, which parallels the one depicted in the mechanism for our experiment.
Zuo, G.; Louie, J. ChemInform 2004, 35.
This paper shows some success using a nickel catalyst for the production of cyclopentanes from vinyl cyclopropanes, and it uses another catalyst, Ir(dF(CF3)ppy)2(dtbbpy)PF6, both of which are used in reactions carried out in paper 2.
Other References
Imiolek, M.; Karunanithy, G.; Ng, W.-L.; Baldwin, A. J.; Gouverneur, V.; Davis, B. G. J. Am. Chem. Soc. 2018, 140, 1568-1571.
M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712-5719.
