Chapter 7: Collection and Analysis of Rate Data
Additional Homework Problems
CDP7-JB
The irreversible liquid-phase reaction
A![]() B + C
B + C
is carried out in a batch reactor. The following data were collected during the course of the reaction:
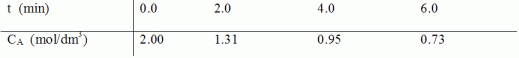
Determine the order of reaction and the specific reaction rate using methods to differentiate your data.
(a) Numerical technique-differentiation formulas.
(Use's to represent these points on any graphs you make.)
Graphical technique-equal area differentiation. (Use O's to represent these points on any graphs you make.)
Differentiating a polynomial. (use x for these points)
(b)Determine the reaction order.
(c)Assume a rate law of the form
-rA![]()
Integrate the equation for the combined mole balance and rate law and then use nonlinear least-squares analysis to determine ![]() and k.
and k.
(d)Where would you place additional data points?