|

|
Electronic Structure of Ferrous Heme Nitroxyls
HNO is a biological effector molecule of growing significance that is proposed to act both as a signaling molecule
in mammals, but also as a drug that elevates the detrimental effects of heart attack and stroke by preventing reperfusion injuries. Although
the endogenous production of HNO has never been shown rigorously, chemists have discovered a number of pathways that nature could use to
produce this molecule from NO, many of them involving transition metal centers. In addition, in its potential role as a signaling molecule,
transition metal centers, especially heme and non-heme iron sites, are prime candidates as HNO receptors. In this regard, it is also
noteworthy that soluble guanylate cyclase (sGC) has been proposed to be activated by HNO.
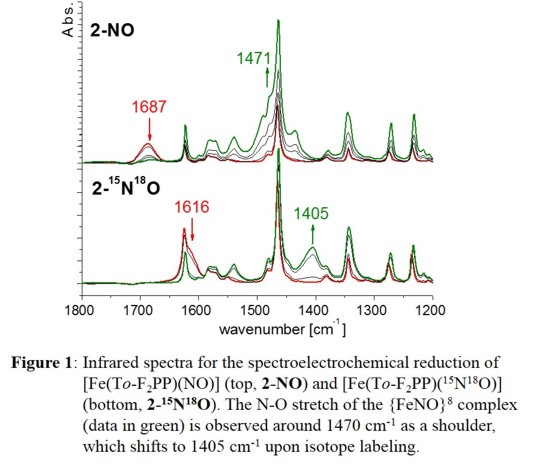
Because of HNO's emerging role as an important effector molecule in biology, there is great current interest in the coordination chemistry
of HNO and its deprotonated form, the nitroxyl anion (NO-), with hemes. We investigated four new ferrous heme-nitroxyl model
complexes, {FeNO}8 in the Enemark-Feltham notation [1],
using three electron-poor porphyrin ligands and the bis-picket fence
porphyrin H2[3,5-Me-BAFP] (3,5-Me-BAFP2- = 3,5-methyl-bis(aryloxy)-fence porphyrin dianion). Electrochemical reduction
of [Fe(To-F2PP)(NO)], for example, induces a shift of n(N-O) from 1687 to 1473 cm-1, indicative
of formation of [Fe(To-F2PP)(NO)]-, as shown in Figure 1. and similar results are obtained with the other hemes as shown in the following Table:
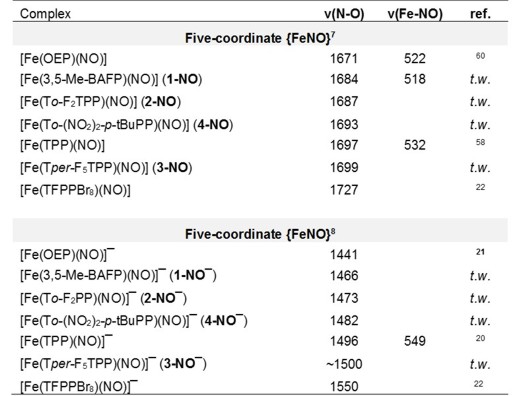
Since the SOMO is responsible for the thermodynamic n-trans effect in ferrous heme-nitrosyls (see ferrous heme nitrosyls),
this could be further probed by one-electron reduction of the complexes, leading to a double-occupation of the SOMO (which becomes the HOMO in the reduced complex) and formation of ferrous nitroxyl,
Fe(II)-NO(-) or {FeNO}8, complexes. However, this is only meaningful if the reduction leads to minimal electronic relaxation, i.e. the composition of the key Fe-N-O bonding MOs, especially the SOMO,
does not change in the reduced state. Surprisingly, this is in fact the case: as shown in Figure 2, there is a strong correlation between the N-O stretching frequencies along our diverse series of
{FeNO}7/{FeNO}8 couples.
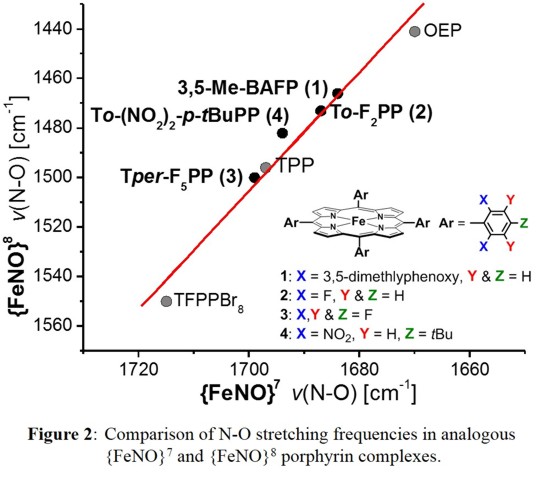
This implies that electronic differences between different {FeNO}7 complexes are 'carried over' into the corresponding {FeNO}8 systems, which requires minimal electronic relaxation
of Fe-NO bonding MOs upon reduction. DFT calculations support this finding and show identical SOMOs for [Fe(Porph)(NO)] and [Fe(Porph)(NO)]- as shown in Figure 3.
The thermodynamic s-trans effect requires that the SOMO has distinct Fe-NO s-bonding character. Double occupation of this orbital should therefore
amplifies these properties, and leads to a strengthening of the Fe-NO bond, and further increase of the trans effect in the {FeNO}8 complexes.
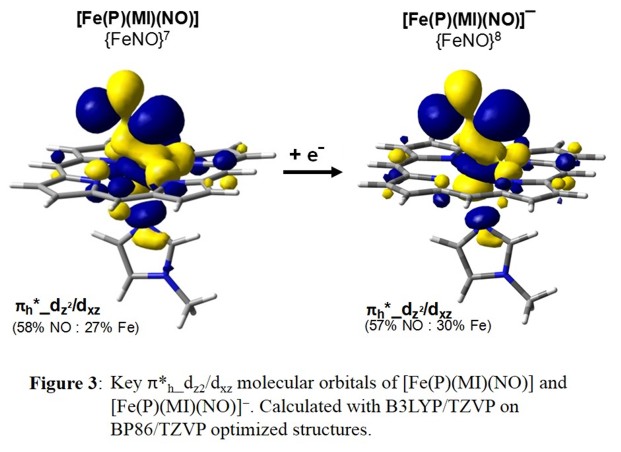
Experimental data verify that this is indeed
the case: resonance Raman studies by Ryan and co-workers show that the Fe-NO stretching frequency increases from 525 cm-1 in [Fe(TPP)(NO)] (in THF) to 549 cm-1 in the reduced {FeNO}8 complex [2]
(despite the fact that reduction increases the Coulomb repulsion between the now double occupied p*h orbital of NO and dxz of iron, which counteracts
this effect), directly demonstrating that the SOMO is Fe-NO bonding in nature. Furthermore, the binding constant of MI in trans position to NO drops by several orders of magnitude in the reduced
{FeNO}8 complexes, directly confirming that the SOMO is in fact responsible for the thermodynamic s-trans effect of NO. In this way, studies on the reduced
iron(II)-nitroxyl model complexes confirm the previous findings about the properties of the SOMO in ferrous heme-nitrosyls. These results could only be obtained with model complexes,
as {FeNO}8 complexes are protonated in an aqueous environment [3].
References:
L. E. Goodrich, S. Roy, E. E. Alp, J. Zhao, M. Y. Hu, N. Lehnert
"Electronic Structure and Biologically Relevant Reactivity of Low-Spin {FeNO}8 Porphyrin Model Complexes: New Insight
from a Bis-Picket Fence Porphyrin"
Inorg. Chem. 2013, 52, 7766-7780
L. E. Goodrich, N. Lehnert
"The trans effect of nitroxyl (HNO) in ferrous heme systems: Implications for soluble guanylate cyclase activation by HNO"
J. Inorg. Biochem. 2013, 118, 179-186
(Special issue: HNO)
A. L. Speelman, N. Lehnert
"Heme versus Non-Heme Iron-Nitroxyl {FeN(H)O}8 Complexes: Electronic Structure and Biologically Relevant Reactivity"
Acc. Chem. Res. 2014, 47, 1106-1116
A. P. Hunt, N. Lehnert
"Heme-Nitrosyls: Electronic Structure Implications for Function in Biology"
Acc. Chem. Res. 2015, 48, 2117-2125
(Special issue: Synthesis in Biological Inorganic Chemistry)
C. van Stappen, L. E. Goodrich, N. Lehnert
"The Interaction of HNO with Transition Metal Centers and Its Biological Significance. Insight into Electronic Structure from
Theoretical Calculations";
in: "The Chemistry and Biology of Nitroxyl (HNO)"; Doctorovich, F.; Farmer, P. J.; Marti, M. A., Eds.,
Elsevier 2017, page 155-192 (Chapter 8)
Literature:
[1] In the Enemark-Feltham notation, {FeNO}n, the index n is equivalent to the number of
valence electrons (= metal(d) plus NO(p*) electrons) of the complex. For example, a complex between Fe(II) and NO
would therefore be classified as {FeNO}7, with formally six Fe(d) and one NO(p*) electrons. See:
Enemark, J. H.; Feltham, R. D. Coord. Chem. Rev. 1974, 13, 339-406.
[2] Choi, I.-K.; Liu, Y.; Feng, D.; Paeng, K.-J.; Ryan, M. D. Inorg. Chem. 1991, 30, 1832-1839.
[3] Kumar, M. R.; Fukuto, J. M.; Miranda, K. M.; Farmer, P. J. Inorg. Chem. 2010, 49, 6283-6292.
|





