|

|
Electronic Structure of Ferrous Heme Nitrosyls
This project aims at elucidating the differences in the spectroscopic properties and electronic structures of five-
and six-coordinate Fe(II)-porphyrin NO adducts, or {FeNO}7 complexes in the
Enemark-Feltham notation [1],
as shown in Scheme 1. This is not only of fundamental interest for the field of heme-nitrosyls (e.g.
the binding of NO to the biological NO sensor soluble guanylate cyclase, etc.), but is also important for the ongoing synthesis of sophisticated models
for bacterial nitric oxide reductase (NorBC) [2].

From the literature, it is known for a long time that the binding of an axial N-donor ligand has a profound effect on the electronic
structure of the Fe(II)-NO unit as evidenced by a strong change of the EPR spectrum [3]. It is also known that the sixth ligand is only
weakly bound in these complexes [4]-[6], but not much quantitative information is available on this topic. We started this project with
binding studies
of N-donor ligands L to five-coordinate [Fe(TPP*)(NO)] (1*, TPP* = tetraphenylporphyrin type ligand) yielding
the six-coordinate complexes [Fe(TPP*)(L)(NO)] (2*):
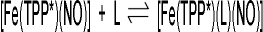
The obtained binding constants show that the interaction of the nitrogen donor ligands with iron(II) trans to NO is weak:
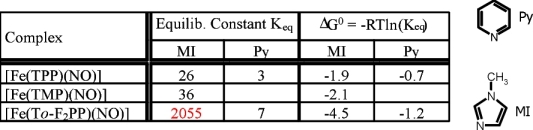
Both the obtained binding constants Keq and the observed degree of denitrosylation in solutions of 1* with excess base
(quantitatively determined from
1H-NMR spectroscopic investigations)
strongly depend on the nature of the porphyrin substituents. Interestingly,
usage of the electron withdrawing fluoro substituents in To-F2PP leads to a strong stabilization of the adduct with MI as compared to
methyl groups in TMP or unsubstituted TPP. On the other hand, fluoro substitution has a distinctively different effect on Keq for MI and Py:
compared to TPP, usage of To-F2PP leads to an increase in binding constant for MI by a factor of 80, whereas for Py the increase is only by a factor
of 2.3. This is attributed to the fact that MI has π-donor properties whereas Py is a π-acceptor.
Hence, the withdrawal of electron density from the porphyrin leads to an increase in binding for MI due to electronic effects, whereas the Py adduct only
experiences a small stabilization due to steric and/or electrostatic effects.
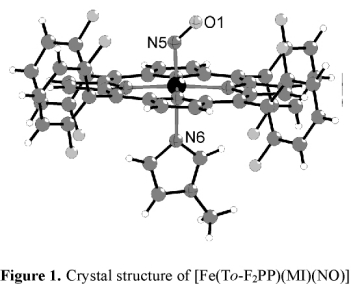
In order to further evaluate this result, we isolated complex [Fe(To-F2PP)(MI)(NO)] (2-F) as a solid and determined the crystal
structure of this compound as shown in Figure 1. However, the structure does not provide any further insight into the
increase in binding constant for MI in this case.
The difference
in electronic structures between 1* and 2* is further investigated using [Fe(TPP)(NO)] (1) and
[Fe(TPP)(MI)(NO)] (2, MI = 1-methylimidazole) as examples. Application of vibrational spectroscopy (IR, resonance Raman and, most recently,
nuclear resonance vibrational spectroscopy (see below))
coupled to isotope substitution allows for the identification of the vibrations of the Fe(II)-NO subunit in 1 and 2.
Quantitative insight into the change of the Fe-NO and N-O bond strengths is not available from a comparison of vibrational frequencies
due to mode mixing. This is especially true for the low-energy Fe-NO stretch, which shows extensive mixing with the Fe-N-O bending mode.
Hence, a determination of the corrersponding force constants is required, which are available from normal coordinate analysis (NCA). However, due to the
size of the molecules investigated here, this is only possible using the
Quantum-Chemistry centered Normal Coordinate Analysis (QCC-NCA)
which we have developed. From
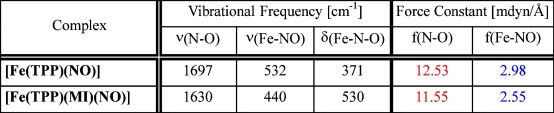
normal coordinate analysis, force constants are calculated to be 12.53 (N-O) and 2.98 mdyn/Å (Fe-NO)
for 1 compared to 11.55 (N-O) and 2.55 mdyn/Å (Fe-NO) for 2 as listed in the Table above.
The direct correlation of the N-O and Fe-NO bond strengths in these
systems is different from corresponding Fe(II)-carbonyls, where an inverse correlation of the C-O and Fe-CO vibrations is found. The tendencies
observed for the nitrosyls show that the binding of an axial N-donor ligand weakens the Fe-NO
σ bond. This is further evidenced by magnetic circular dichroism (MCD)
spectroscopy, which indicates a dramatic decrease of the spin population on iron upon the binding of MI.
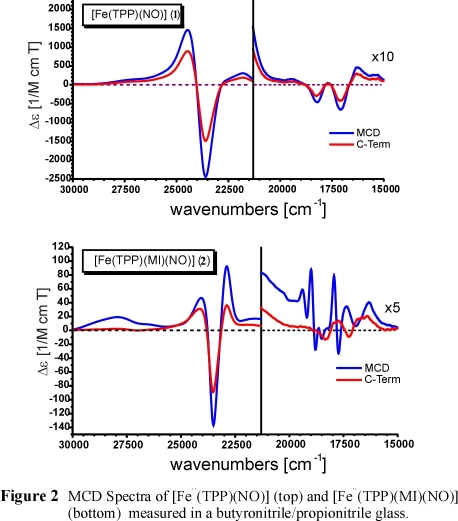
MCD intensity is generally considered to arise from three different mechanisms
(cf. MCD page). The C-term is temperature dependent and originates from spin-orbit coupling
of the ground and target excited states with other intermediate excited states. On the other hand, A- and B-terms are temperature independent
and are also present in diamagnetic materials. Thus, the paramagnetic (C-term) contribution to the total spectrum can be extracted by substracting
MCD data taken at variable temperatures. Figure 2 shows the MCD spectra of five-coordinate (5C) [Fe(TPP)(NO)] (top) and of
six-coordinate (6C) [Fe(TPP)(MI)(NO)] (bottom) in comparison. As one can see, these data are very different.
In the case of 1, the C-term spectrum obtained from temperature-dependent data
is identical in appearance to the total spectrum. Consequently, the MCD response is dominated by the paramagnetic C-term contribution.
Since spin-orbit coupling is weak for light elements like carbon, nitrogen and oxygen, the C-term nature of the spectrum indicates that a significant
amount of spin-density of the unpaired electron in 1 must be located on the formally iron(II) center. This is dramatically different for the 6C
complex 2. From Figure 2, bottom, one can see that the deconvoluted MCD C-term spectrum is different from the total spectrum,
which is in fact dominated by temperature-independent diamagnetic contributions (A-term and B-term). These are generally observed for
diamagnetic metal porphyrin complexes due to the occurrence of (practically) degenerate excited states in the porphyrin dianion with
approximate D4h symmetry. This indicates that the unpaired spin density is mostly located on the NO unit in the 6C case,
which leads to the temperature-independent MCD spectrum of a diamagnetic low-spin Fe(II)-porphyrin.
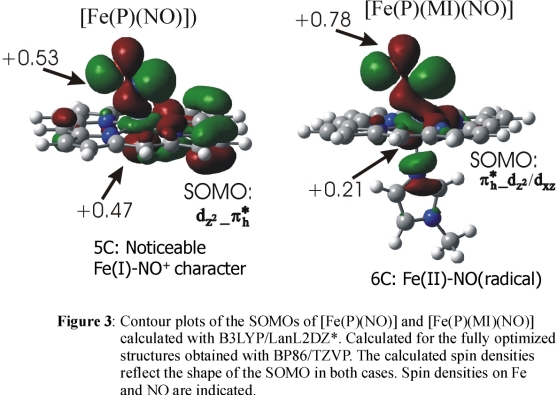
DFT calculations show that in the
case of 1, a strong Fe-NO σ bond mediated by the singly occupied π* orbital
of NO and the empty dz2 orbital of low-spin Fe(II)
is present (cf. Figure 3) leading to a large transfer of spin density from the NO ligand to Fe(II) corresponding to an electronic structure with noticeable
Fe(I)-NO+ character. On coordination of the sixth ligand, the spin density is pushed back from the iron toward the NO ligand
as shown in Figure 3
resulting in an Fe(II)-NO(radical) type electronic structure in agreement with the MCD result. This corresponds to a weakening of the
Fe-NO σ bond in agreement with the experimental force constants. Importantly, the increased amount of spin density on NO in the
six-coordinate case is advantageous for the proposed trans mechanism of bacterial NO reductases
(NorBC; see bioinorganic page),
which requires radical coupling of
two molecules of NO forming N2O.
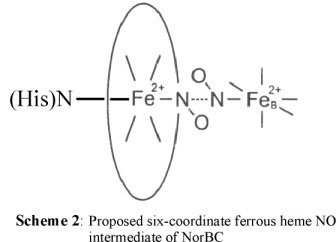
We have therefore proposed that if NO reduction in NorBC indeed follows the trans mechanism, then the reactive intermediate should be a six-coordinate ferrous heme
NO complex, as shown in Scheme 2.
In this context, it should also be noted that hemoglobin and myoglobin form six-coordinate nitrosyl adducts, and therefore, this is definitively a mechanistic
possibility for NorBC. In addition, shortly after our paper was published, it was demonstrated that the corresponding intermediate
in cytochrome c oxidase (CCO) shows in fact a six-coordinate heme-nitrosyl [7].
Nuclear Resonance Vibrational Spectroscopy (NRVS) is an ideal method to identify metal-ligand vibrations and improve the accuracy of vibrational analyses,
due to the fact that NRVS intensities can be directly calculated from NCA simulations. We therefore applied this method
to interrogate the vibrational properties (and electronic structures)
of five- and six-coordinate ferrous heme-nitrosyls. In particular, we used oriented single-crystal NRVS on the six-coordinate model complex
[Fe(TPP)(MI)(NO)] combined with 15N18O isotope labeling and QCC-NCA simulations of our data (in collaboration with
Prof. Bob Scheidt, University of Notre Dame).
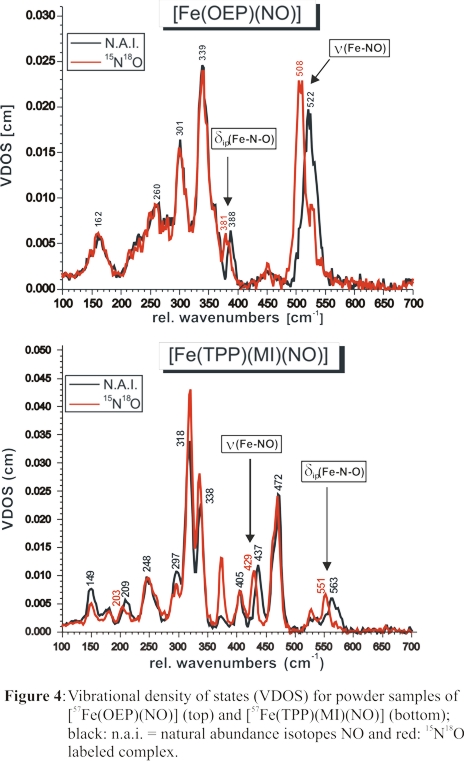
Two isotope-sensitive features at 437 and 563 cm-1 are identified from these data as shown in Figure 4, bottom. To assign these bands, the vibrational energies
and isotope shifts of these features were simulated using our QCC-NCA approach. Accounting for the strong out-of-plane polarization of the 437 cm-1
feature from single-crystal NRVS measurements, ν(Fe-NO) was unambiguously assigned to the band at 437 cm-1, and
δip(Fe-N-O) was identified with the feature at 563 cm-1. In this way, NRVS measurements have resolved a long-standing
controversy in the literature about the assignment of ν(Fe-NO) in 6C ferrous heme-nitrosyls in proteins and model complexes. The significant drop in the
Fe-NO stretching freqiency between five- and six-coordinate ferrous heme-nitrosyls evident from Figure 4 illustrates again the strong σ trans
effect of NO.
Finally, we have designed a tailed porphyrin complex, shown in Scheme 3, that allows for the generation of a truly six-coordinate ferrous heme-nitrosyl complex
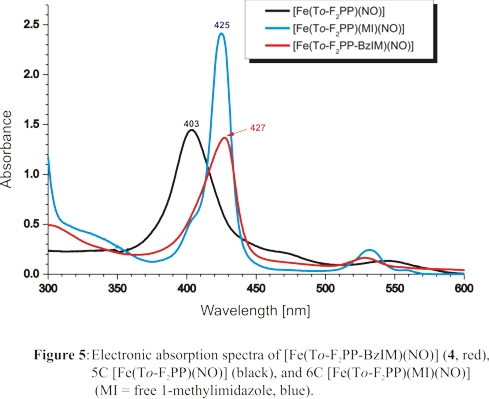
in solution at room temperature. This is illustrated by the UV-Vis spectrum of this complex, taken at room temperature, shown in red in Figure 5. This spectrum is
in close agreement with that of the complex [Fe(To-F2PP)(MI)(NO)] (blue), which is obtained in the presence of excess MI.
We have conducted further investigations on the one-electron reduced forms of ferrous heme nitrosyl complexes, {FeNO}8 in the Enemark-Feltham notation. These ferrous heme nitroxyl complexes
are relevant in the context of the biological chemistry of HNO. As it turns out, heme {FeNO}8 complexes also provide clues with respect to the electronic structures
of their {FeNO}7 precursors. These results are briefly summarized here.
References:
V. K. K. Praneeth, F. Neese, N. Lehnert
"Spin Density Distribution in Five- and Six-coordinate Iron(II)-Porphyrin NO Complexes evidenced by
Magnetic Circular Dichroism Spectroscopy"
Inorg. Chem. 2005, 44, 2570-2572
V. K. K. Praneeth, C. Näther, G. Peters, N. Lehnert
"Spectroscopic Properties and Electronic Structure of Five- and Six-Coordinate Iron(II)-Porphyrin
NO Complexes: Effect of the axial N-Donor Ligand"
Inorg. Chem. 2006, 45, 2795-2811
N. Lehnert, V. K. K. Praneeth, F. Paulat
"Electronic Structure of Fe(II)-Porphyrin Nitroxyl Complexes:
Molecular Mechanism of fungal Nitric Oxide Reductase (P450nor)"
J. Comput. Chem. 2006, 27, 1338-1351
(special issue: Computational Bioinorganic Chemistry)
F. Paulat, T. C. Berto, S. DeBeer George, L. Goodrich, V. K. K. Praneeth, C. D. Sulok, N. Lehnert
"The Vibrational Assignments of Six-Coordinate Ferrous Heme Nitrosyls: New Insight from Nuclear Resonance
Vibrational Spectroscopy"
Inorg. Chem. 2008, 47, 11449-11451
T. C. Berto, V. K. K. Praneeth, L. E. Goodrich, N. Lehnert
"Iron-Porphyrin NO Complexes with Covalently Attached N-Donor Ligands:
The Formation of a Stable Six-Coordinate Species in Solution"
J. Am. Chem. Soc. 2009, 131, 17116-17126
N. Lehnert, M. G. I. Galinato, F. Paulat, G. B. Richter-Addo, W. Sturhahn, N. Xu, J. Zhao
"Nuclear Resonance Vibrational Spectroscopy applied to [Fe(OEP)(NO)]: the Vibrational Assignments of
Five-Coordinate Ferrous Heme Nitrosyls and Implications for Electronic Structure"
Inorg. Chem. 2010, 49, 4133-4148
L. E. Goodrich, F. Paulat, V. K. K. Praneeth, N. Lehnert
"Electronic Structure of Heme-Nitrosyls and Its Significance for Nitric Oxide Reactivity, Sensing,
Transport, and Toxicity in Biological Systems"
Inorg. Chem. 2010, 49, 6293-6316
(Inorganic Chemistry FORUM: The Coordination Chemistry of Nitric Oxide and its Significance for Metabolism,
Signaling and Toxicity)

N. Lehnert, J. T. Sage, N. Silvernail, W. R. Scheidt, E. E. Alp, W. Sturhahn, J. Zhao
"Oriented Single-Crystal Nuclear Resonance Vibrational Spectroscopy of [Fe(TPP)(MI)(NO)]: Quantitative
Assessment of the trans Effect of NO"
Inorg. Chem. 2010, 49, 7197-7215
L. E. Goodrich, S. Roy, E. E. Alp, J. Zhao, M. Y. Hu, N. Lehnert
"Electronic Structure and Biologically Relevant Reactivity of Low-Spin {FeNO}8 Porphyrin Model Complexes: New Insight
from a Bis-Picket Fence Porphyrin"
Inorg. Chem. 2013, 52, 7766-7780
T. C. Berto, N. Xu, S. R. Lee, A. J. McNeil, E. E. Alp, J. Zhao, G. Richter-Addo, N. Lehnert
"Characterization of the Bridged Hyponitrite Complex {[Fe(OEP)]2(μ-N2O2)}:
Reactivity of Hyponitrite Complexes and Biological Relevance"
Inorg. Chem. 2014, 53, 6398-6414
(Inorganic Chemistry FORUM: Insights into Spectroscopy and Reactivity from Electronic Structure Theory)
N. Lehnert, W. R. Scheidt, M. W. Wolf
"Structure and Bonding in Heme-Nitrosyl Complexes and Implications for Biology"
Struct. Bond. 2014, 154, 155-224
(Special issue: Nitrosyl Complexes in Inorganic Chemistry, Biochemistry and Medicine II)
Literature:
[1] In the Enemark-Feltham notation, {FeNO}n, the index n is equivalent to the number of
valence electrons (= metal(d) plus NO(π*) electrons) of the complex. For example, a complex between Fe(II) and NO
would therefore be classified as {FeNO}7, with formally six Fe(d) and one NO(π*) electrons. See:
Enemark, J. H.; Feltham, R. D. Coord. Chem. Rev. 1974, 13, 339-406.
[2] Hunt, A. P.; Lehnert, N. Acc. Chem. Res. 2015, 48, 2117-2125.
[3] 'Nitric Oxide Research from Chemistry to Biology: EPR Spectroscopy of Nitrosylated Compounds';
Henry, Y. A.; Guissani, A.; Ducastel, B., eds., R. G. Landes Company, Austin, 1997.
[4] Wyllie, G. R. A.; Schulz, C. E.; Scheidt, W. R. Inorg. Chem. 2003, 42, 5722-5734.
[5] Patchkovskii, S.; Ziegler, T. Inorg. Chem. 2000, 39, 5354-5364.
[6] Zhang, Y.; Gossman, W.; Oldfield, E. J. Am. Chem. Soc. 2003, 125, 16387-16396.
[7] Pinakoulaki, E.; Ohta, T.; Soulimane, T.; Kitagawa, T.; Varotsis, C. J. Am. Chem. Soc. 2005, 127, 15161-15167.
|












