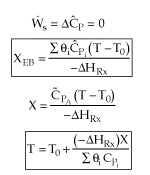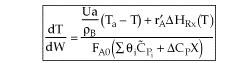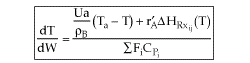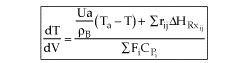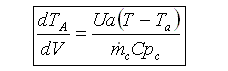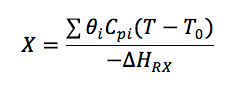Chapter 11: Nonisothermal Reactor Design: The Steady State Energy Balance and Adiabatic PFR Applications
Topics
- Why Use the Energy Balance?
- Overview of User Friendly Energy Balance Equations
- Manipulating the Energy Balance, DHRx
- Reversible Reactions
- Adiabatic Reactions
- Interstage Cooling/Heating
| Energy Balances, Rationale and Overview | top |
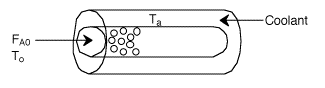
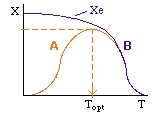
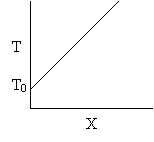
Let's calculate the volume necessary to achieve a conversion, X, in a PFR for a first-order, exothermic reaction carried out adiabatically. For an adiabatic, exothermic reaction the temperature profile might look something like this:
The combined mole balance, rate law, and stoichiometry yield:
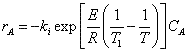 |
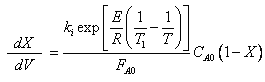 |
To solve this equation we need to relate X and T.
We will use the Energy Balance to relate X and T. For example, for an adiabatic reaction, e.g.,![]() , in which no inerts the energy balance yields
, in which no inerts the energy balance yields
![]()
We can now form a table like we did in Chapter 2,

| User Friendly Energy Balance Equations | top |
The user friendly forms of the energy balance we will focus on are outlined in the following table.
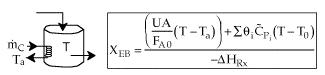
User friendly equations relating X and T, and Fi and T 1. Adiabatic CSTR, PFR, Batch, PBR achieve this:
2. CSTR with heat exchanger, UA(Ta-T) and large coolant flow rate.
3A. In terms of conversion, X
3B. In terms of molar flow rates, Fi
4. For Multiple Reactions
5. Coolant Balance
|
||||||||||||||
These equations are derived in the text. These are the equations that we will use to solve reaction engineering problems with heat effects. |
| Energy Balance | top |
In the material that follows, we will derive the above equations.
We need to put the above equation into a form that we can easily use to relate X and T in order to size reactors. To achieve this goal, we write the molar flow rates in terms of conversion and the enthalpies as a function of temperature. We now will "dissect" both Fi and Hi. [Note: For an animated derivation of the following equations, see the Interactive Computer Modules (ICMs) Heat Effects 1 and Heat Effects 2.]
Flow Rates, Fi |
||
For the generalized reaction:
|
||
In general,
|
||
Assuming no phase change: |
||
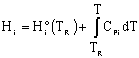 |
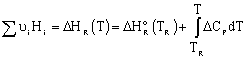 |
|
|
||
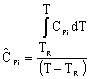 |
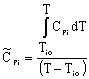 |
|
|
||
 |
||
|
||
Adiabatic Energy Balance: |
|
 | |
Adiabatic Energy Balance for variable heat capacities: |
|
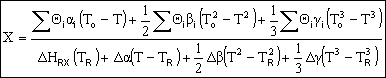
For constant heat capacities:
We will only be considering constant heat capacities for now. | |
| Reversible Reactions | top |
Consider the reversible gas phase elementary reaction.
![]()
The rate law for this gas phase reaction will follow an elementary rate law.
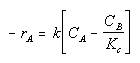
Where Kc is the concentration equilibrium constant. We know from Le Chaltlier's Law that if the reaction is exothermic, Kc will decrease as the temperature is increased and the reaction will be shifted back to the left. If the reaction is endothermic and the temperature is increased, Kcwill increase and the reaction will shift to the right.
at equilibrium
$$ -r_{A} = 0 $$
$$ K_{c} = \frac{C_{B\beta}}{C_{A\beta}} = \frac{C_{A0}X_{\beta}{\frac{T_{0}}{T}}p}{C_{A0}(1 - X_{\beta}){\frac{T_{0}}{T}}p} = \frac{X_{\beta}}{1 - X_{\beta}}$$
$$ X_{\beta} = \frac{K_{c}}{1+K_{c}} $$
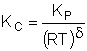
|
Van't Hoff Equation
|
For the special case of Integrating the Van't Hoff Equation gives: |
|
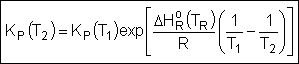
|
|
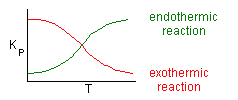
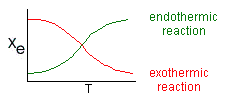
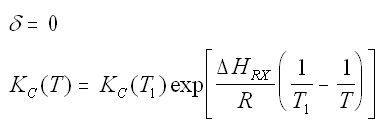
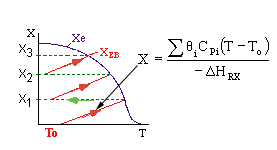
Adiabatic Equilibrium
Conversion on Temperature
Exothermic ΔH is negative
Adiabatic Equilibrium temperature (Tadia) and conversion (Xeadia
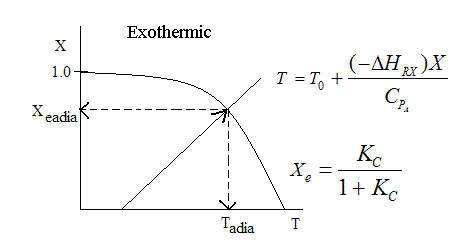
Endothermic ΔH is positive
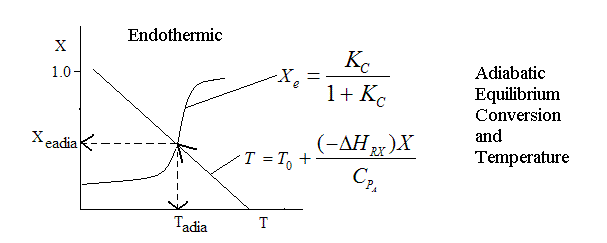
| Adiabatic Reactions | top |
Algorithm Adiabatic Reactions:
Suppose we have the Gas Phase Reaction
![]()
that follows an elementary rate law. To generate a Levenspiel plot to size CSTRs and PFRs we use the following steps or as we will see later use POLYMATH.
| 1. Choose X | |
| Calculate T | |
| Calculate k | 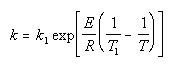
|
| Calculate KC | 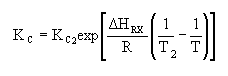
|
| Calculate To/T | |
| Calculate CA | 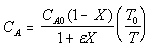
|
| Calculate CB | 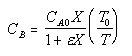
|
| Calculate -rA | 
|
| 2. Increment X and then repeat calculations. | |
| 3. When finished, plot |
|
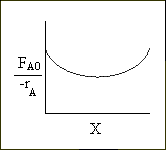
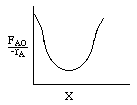 Levenspiel Plot for an exothermic, adiabatic reaction. We can now use the techniques developed in Chapter 2 to size reactors and reactors in series to compare and size CSTRs and PFRs. |
|
Consider: 
|
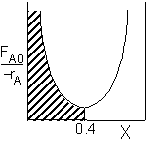
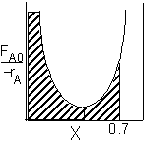
|
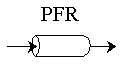 PFR Shaded area is the volume. PFR Shaded area is the volume. |
||
For an exit conversion of 40% |
For an exit conversion of 70% |
|
|
|
|
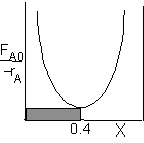
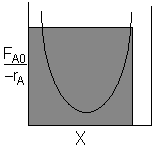
| CSTR |
||
For an exit conversion of 40% |
For an exit conversion of 70% |
|
|
|
|
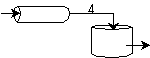
We see for 40% conversion very little volume is required. CSTR+PFR |
||
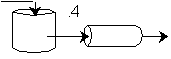
|
|
|
| (a) | (b) | |
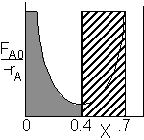
For an intermediate conversion of 40% and exit conversion of 70% |
||
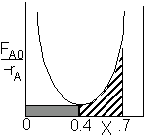
|
|
|
| (a) | (b) | |
Looks like the best arrangement is a CSTR with a 40% conversion followed by a PFR up to 70% conversion.
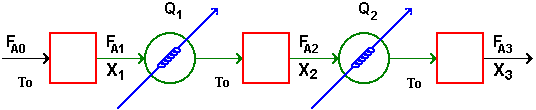
| Interstage Cooling/Heating | top |
|
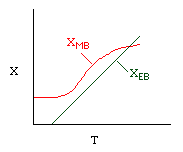
Curve A: Reaction rate slow, conversion dictated by rate of reaction and reactor volume. As temperature increases rate increases and therefore conversion increases. |
||
Interstage Cooling: |
||
|
||
|
||
CSTR Algorithm
1.) |
Given X |
|
2.) |
Given T |
|
3.) |
Given V |
|
 |
||