|

|
Bioinorganic Chemistry
Research projects that are currently pursued in my group relate to the biological nitric oxide (NO) metabolism;
i.e. the synthesis, function and degradation of nitric oxide in the biosphere. Nitric oxide is a poisonous gas,
which, however, has proven to be of great biological significance. In 1992, it was therefore voted as
'the molecule of the year' by the magazine Science [1]. These pioneering results triggered further research and up to this day,
it is known that NO plays a key role in nerve signal transduction, vasodilation, blood clotting and immune response by white blood cells.
New biological functions of NO and the corresponding, one electron reduced nitroxyl ion are still discovered. Many of the biologically
important reactions of nitric oxide are mediated by heme proteins. NO is produced in vivo by the nitric oxide synthase (NOS) family of
enzymes. The cardiovascular regulation by NO (produced by endothelial(e-) NOS) is then mediated by soluble guanylate cyclase (sGC),
which is activated by coordination of NO to its ferrous heme active site. In addition, the role of nitric oxide in vasodilation is
exploited by certain blood-sucking insects that inject NO into the bites of their victims using small NO-carrier heme proteins, the
so-called Nitrophorins (Np) [2].
Our research focuses on model systems for
denitrifying enzymes,
especially the nitrite (NO2-) and nitric oxide
reductases. In addition, we are also interested in scavenging NO reductases that are found in certain pathogenic
bacteria. The major goal of this research is the investigation of the different mechanisms of activation of
NO when coordinated to transition metal centers (especially heme and non-heme iron). In this respect, we synthesize
model systems using Schlenk techniques and determine their
spectroscopic and electrochemical properties. This information is then correlated to the experimentally observed
reactivities of these systems and the postulated mechanisms of the enzymatic reactions using quantum-chemical
calculations. The synthetic work mainly focuses on various non-heme (especially polypyridyl and related ligands, and
hydrotris(pyrazolyl)borates, etc.) and porphyrin ligands, their transition metal complexes
and corresponding NO adducts as well as studies into their reactivity.
We are especially interested in the respiratory nitric oxide reductases (NORs), because these are key enzymes in the
nitrogen cycle responsible
for the generation of the important green house gas and ozone-depleting agent N2O, and the scavenging NORs, because
these are key enzymes in bacterial pathogenesis (since these enzymes provide resistance to these pathogens against the
mammalian immune defense agent NO) [3]. With respect to the respiratory NORs, there are two different classes of enzymes.
Bacterial respiratory NOR (NorBC or cNOR) reduces NO to nitrous oxide (N2O) at
a mixed heme/non-heme iron active site, where the heme shows axial histidine coordination [4],[5].
In comparison, the same reaction is performed by
fungal respiratory nitric oxide reductase (Cyt P450nor) at a single heme active site, which, in contrast, has an axial cysteine ligand [6],[7].
Hence, the bacterial
and fungal enzymes catalyze the same reaction, but utilize different active sites and mechanisms as shown in Figure 1. Central research goals are the elucidation
of the reaction mechanisms of these enzymes and the properties of heme-nitrosyls in general, as a function of porphyrin substitutions and trans-ligands
to NO. To this end, a dual strategy is applied. Firstly, 'simple' model complexes of type [Fe(TPP*)(L)(NO)]n+
(TPP* = tetraphenylporphyrin type ligand;
L = N-donor, thiolate, etc.) are synthesized, which allow for the systematic investigation of the porphyrin substituent and trans-ligand effect on the
coordinated NO. Complementarily, we are working on the synthesis of sophisticated model complexes for both NorBC and P450nor. In contrast to the
respiratory enzymes, the active site of scavenging NORs corresponds to a non-heme diiron core with a flavin cofactor in close proximity (Figure 1) [8].
In this case, we are studying mono- and dinuclear non-heme iron-nitrosyl model complexes to obtain insight into the catalytic mechanism of
these enzymes. We investigate our model compounds using a variety of spectroscopic techniques (see Research
home) in correlation with density functional theory (DFT) calculations. The obtained results are not only
important for the understanding of the mechanisms of these enzymes, but are also relevant for various biological functions of NO as described above.
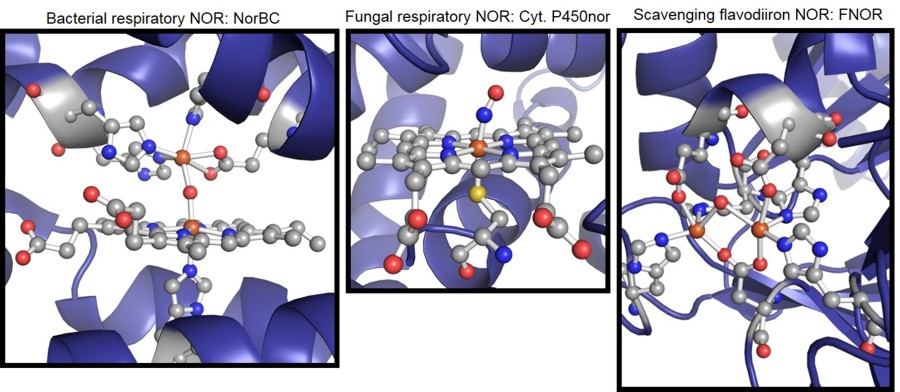
Figure 1. Active site structures of different nitric oxide reductases.
In dissimilatory denitrification, nitric oxide is produced by the reduction of nitrite,
which (amongst others) is performed by a Cu Nitrite Reductase (CuNIR). In collaboration with
Prof. Dr. Kiyoshi Fujisawa (University of Tsukuba, Japan), model studies on
this enzyme are performed using hydrotris(pyrazolyl)borate, tris(pyrazolyl)methane, and bis(pyrazolyl)methane ligands.
In addition, we are developing CuNIR model complexes as electrocatalysts for the production of NO on demand by electrocatalystic reduction of nitrite [9]. Potential
applications for these systems are in catheters with NO-releasing properties (to prevent blood clotting and bacterial biofilm formation) and
inhalation therapy. These studies are performed in collaboration with Prof. Mark Meyerhoff's group (University of Michigan).
Literature:
[1] Snyder, S. H. Science 1992, 257, 494-496.
[2] Hunt, A. P.; Lehnert, N. Acc. Chem. Res. 2015, 48, 2117-2125.
[3] Speelman, A. L.; Lehnert, N. Acc. Chem. Res. 2014, 47, 1106-1116.
[4] Zumft, W. G. J. Inorg. Biochem. 2005, 99, 194-215.
[5] Shiro, Y. Biochim. Biophys. Acta 2012, 1817, 1907-1913.
[6] Daiber, A.; Shoun, H.; Ullrich, V. J. Inorg. Biochem. 2005, 99, 185-193.
[7] McQuarters, A. B.; Wirgau, N. E.; Lehnert, N. Curr. Op. Chem. Biol. 2014, 19, 82-89.
[8] Kurtz, D. M., Jr. Dalton Trans. 2007, 4115-4121.
[9] Qin, Y.; Zajda, J.; Ren, H.; Toomasian, J.; Major, T.; Rojas Pena, A.; Carr, B.; Johnson, T.; Haft, J.; Bartlett, R.;
Hunt, A.; Lehnert, N.; Meyerhoff, M. Mol. Pharmaceutics 2017, 14, 3762-3771.
Research Highlights
 Electronic structure of Ferrous Heme-Nitrosyls
Electronic structure of Ferrous Heme-Nitrosyls
 Electronic structure of Ferric Heme-Nitrosyls
Electronic structure of Ferric Heme-Nitrosyls
 Effect of Axial Thiolate Coordination in Heme-Nitrosyls
Effect of Axial Thiolate Coordination in Heme-Nitrosyls
 Second Coordination Sphere Effects in Copper Complexes
Second Coordination Sphere Effects in Copper Complexes
 Non-heme Iron-Nitrosyl Complexes: Intermediates in bacterial NO Reductases
Non-heme Iron-Nitrosyl Complexes: Intermediates in bacterial NO Reductases
 Modeling Flavodiiron Nitric Oxide Reductases
Modeling Flavodiiron Nitric Oxide Reductases

Prof. K. Fujisawa
|



