Obtaining an Absorbance Spectrum
The Absorbance Spectrum
You've looked at what light does all at once, but for many things, including this experiment, the light is broken down into wavelengths. Each wavelength has it's own color (Found Already!). When you see how each wavelength of light interacts with the solution on its own, you can generate an absorbance spectrum (graph) for your solution.
Each wavelength can absorb differently, below is an example of how that happens.
1). The different colors of light hit the solution one at a time.
2). Each color of light interacts with the molecules and some part of the light is Absorbed, while the rest is Transmitted.
3). The amount of light that is Transmitted passes through the solution to the detector.
4). The machine then can convert the amount of light that is Transmitted into the amount of light that was blocked or Absorbed by the solution.
As the light interacts with the sample, you can ask yourself.
- If i have a red solution, what wavelengths would absorb the least?
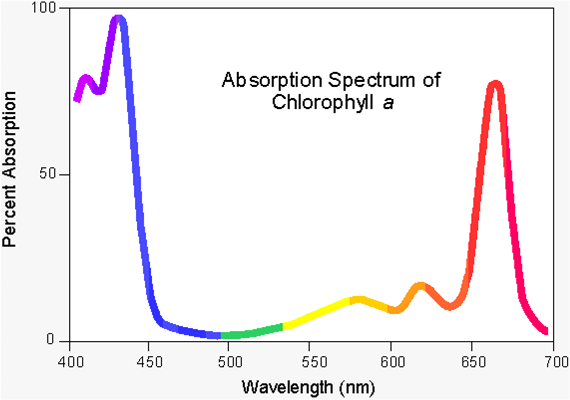
Once the light interacts with your solution, you can graph it, much like this spectrum of Chlorophyll A
Activity not available on mobile devices (description)
A question to think about:
- Look at the spectrum for Chlorophyll A.
- What color is clorophyll?
- Where is that color on the spectrum?
- What is the absorbance at this color?
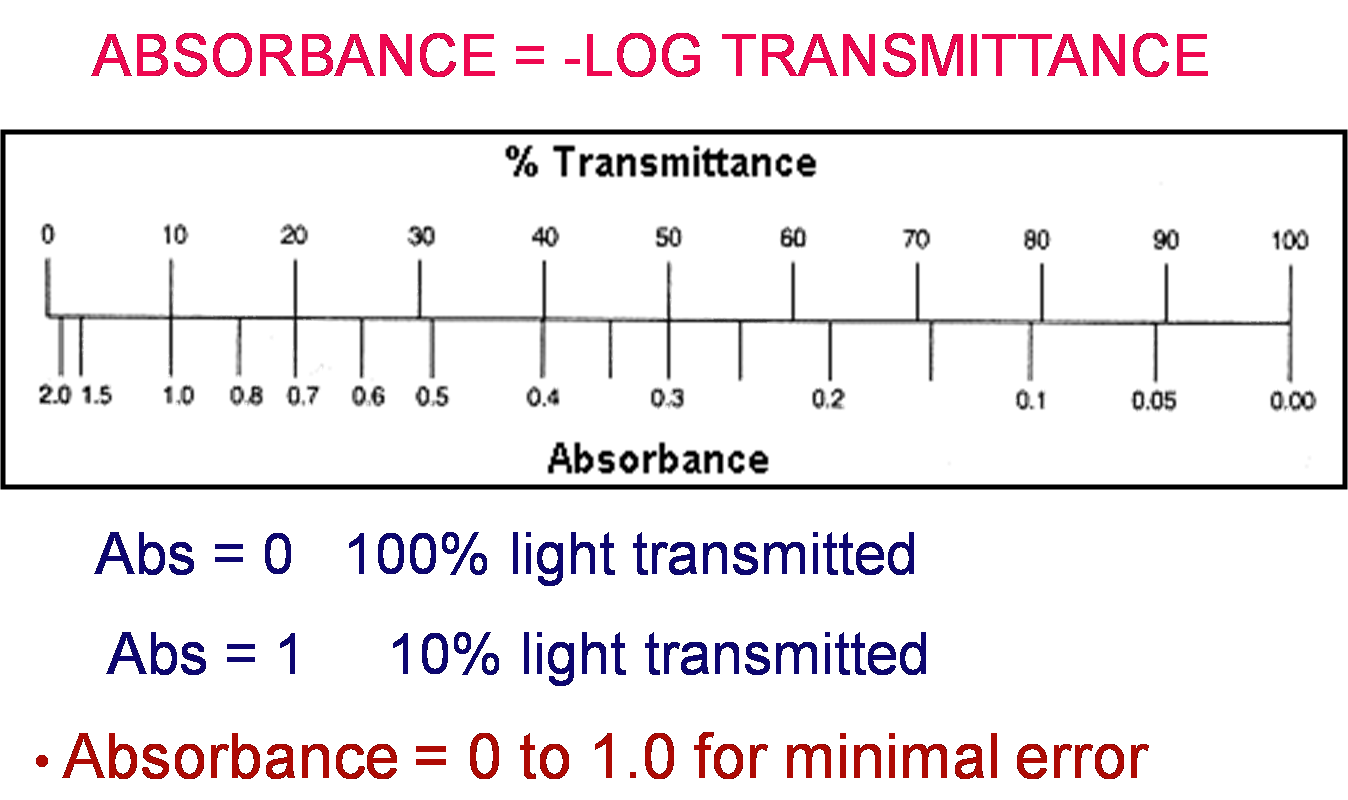
Absorbance vs. Transmittance
Absorbance and Transmittance are related. As one goes up, the other goes down
Absorbance is the amout of light that is blocked by sample molecules
Transmittance is the amout of light that passes through the solution.
But HOW are they related?