Preparing a solution of known concentration
Volume & Concentration
When you're making a solution of a given compound, you need to know what concentration you want, and what volume you want. These are important because they relate to how much of your compound you need.
You will also need to find the molecular weight of the compound like you did on the first page.
The volume of your container is important, because if there is a larger volume, you need more material.
Test yourself!
 Show/hide comprehension question...
Show/hide comprehension question...
The next important step after you select a volume is the concentration
The concentration is the amount of something in a given container.
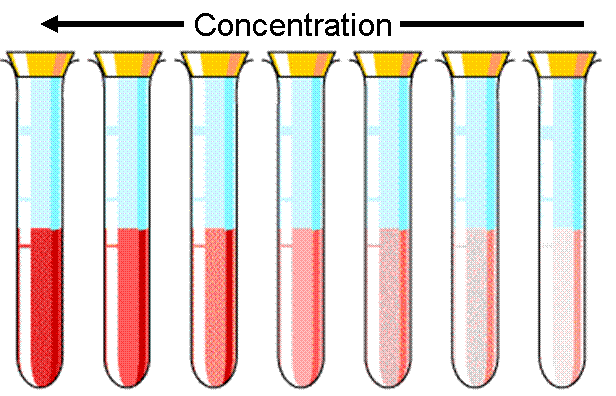
The higher the concentration, the more of the sample there is.
Moving from right to left in this picture the concentration is increasing, and you can see the solutions getting darker.
 Show/hide comprehension question...
Show/hide comprehension question...
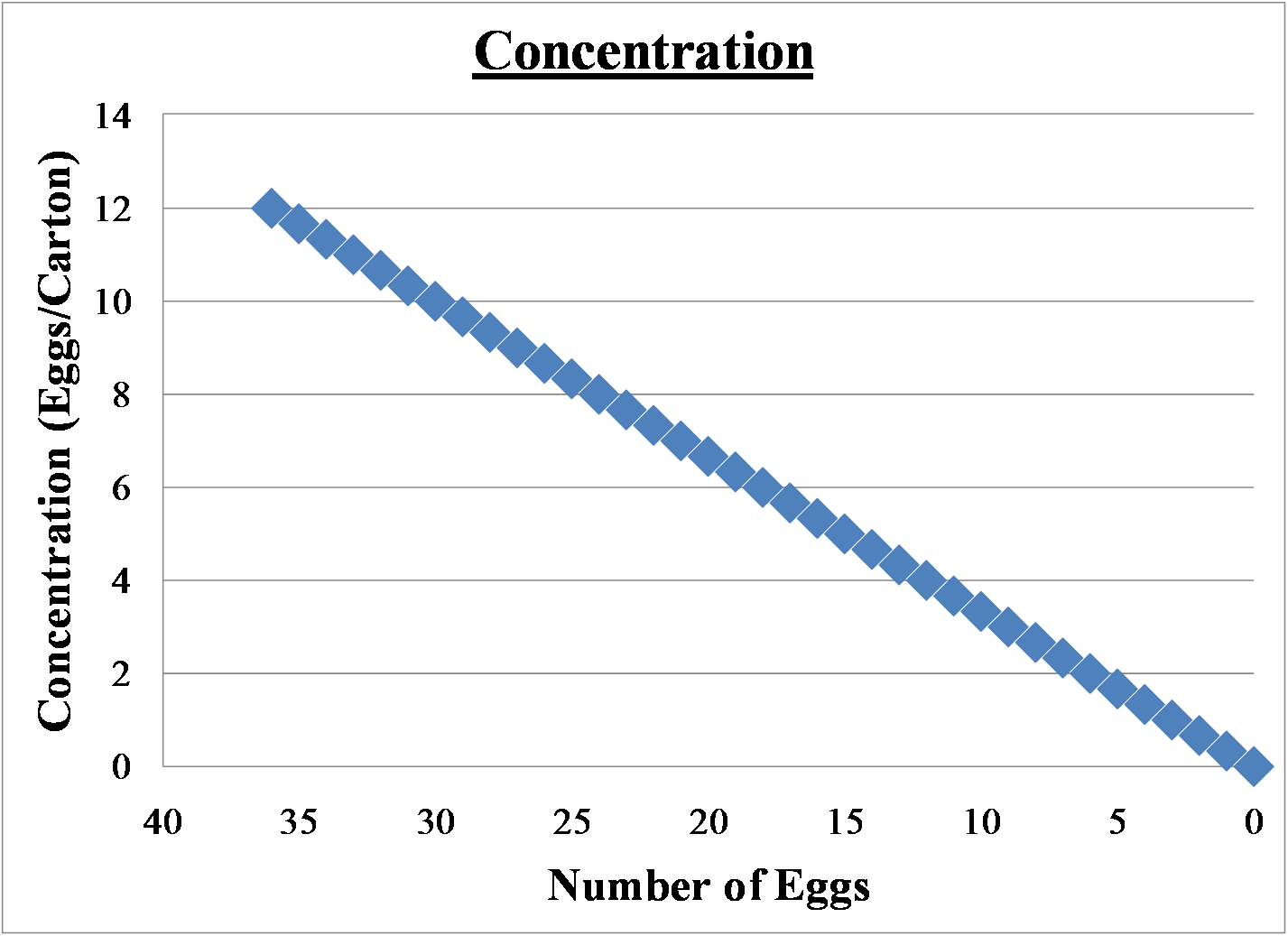
An example of concentration is stated below
There are 36 eggs in 3 cartons, so the concentration is 12eggs/carton
The graph below shows how the concentration changes as you remove eggs from the cartons. There are 3 cartons of 12 eggs to start with. 36eggs/3 cartons. The concentration is 12eggs/carton. As you remove eggs from the cartons, the concentration goes down. There are still 3 cartons, but now less eggs.