Generating and Using a Calibration Graph
Using your Calibration Graph!
Now for the fun part! Using the calibration plot that YOU made from the data two pages ago. We are going to determing the concentration of an unknown solution. Make sure you have your plot ready, because here we go!
Here's a typical problem. You take 3mL of your unknown sample and 7mL water and mix them together. The dilluted sample gives an absorbance of 0.432. What is the concentration of the initial unknown?
Where do you begin?! Well, you have your calibration graph, and it SHOULD look something like this, all properly labeled.
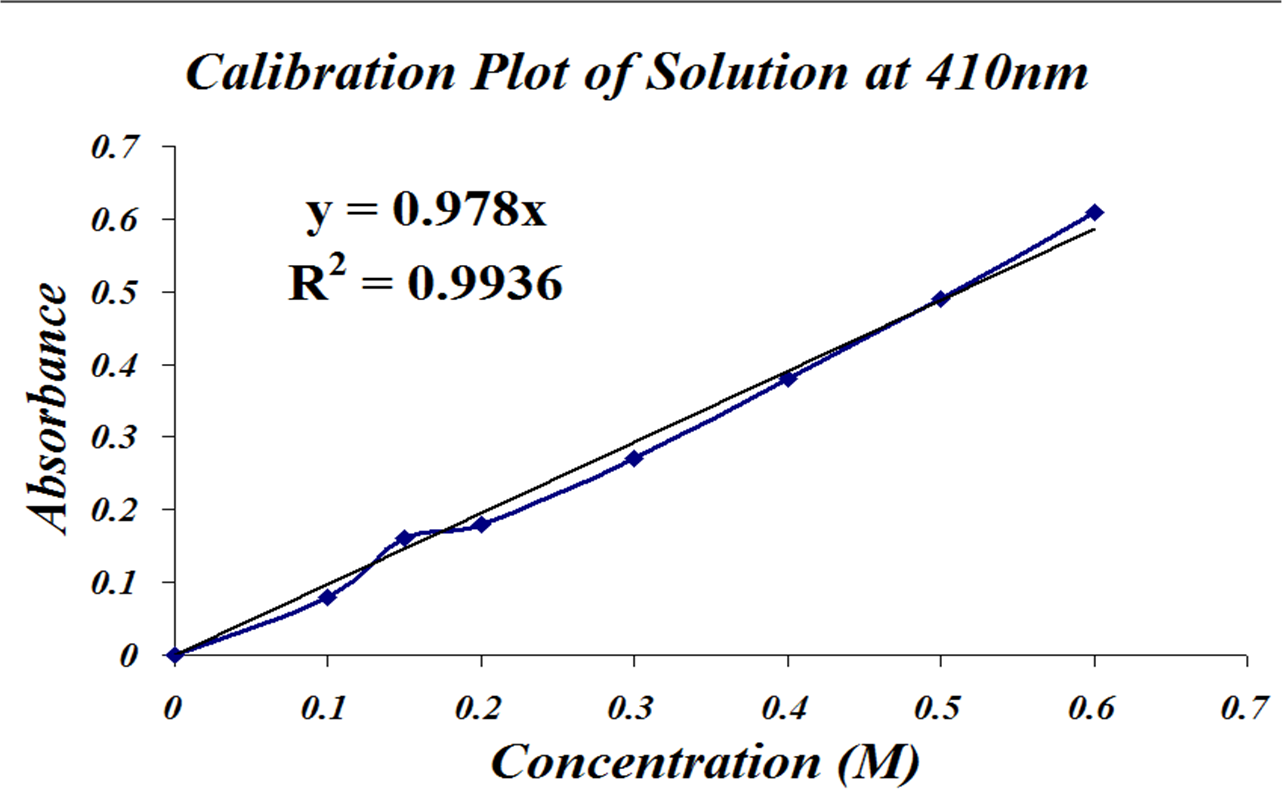
1). You have an absorbance, and you have a straight line equation that relates absorbance to concentration. This is the line of best fit through your data.

2). Now this is the absorbance of your DILUTED solution. But what was the concentration of your ORIGINAL solution? Remember you dilluted it once, so you can use the Dilution Equation

Ready to try one on your own?
Need some practice? Here are a few more problems. Try to figure them out on your own!
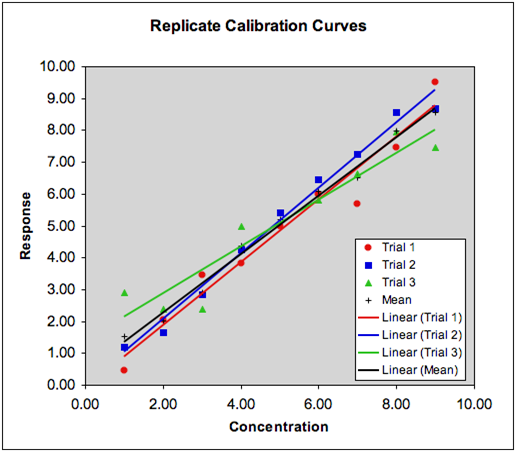
Common Errors In Calibration Plots
- Spectrophotomer is not calibrated
- Abs readings are incorrect
- Diluted samples are prepared incorrectly or contaminated
- Inappropriate wavelength chosen for calibration graph
- The calibration line is not a "best fit" line