Post Experiment Problems
After you finish your experiment, see if you can answer these problems below. These problems are very similar to those that will be found on your exams, and are an indication of if you are understanding the material fully. Make sure to read the questions carefully and think about your answers, as you may see similar problems later on (hint hint!)
Question Set 1
This question set deals with the first part of this tutorial. The preparation and dilution of solutions.
a). You need to prepare 100mL of 0.1M Cu(NO3)2
How many grams of Cu(NO3)2 * 3H2O will you need to weigh out?
b). Instead of using a volumetric flask, a student used a graduated cylinder to add 100mL of water to a beaker containing the solid. The resulting solution was too concentrated. Why?
c), You add 5 mL of 0.10 M Cu(NO3)2 to 15 mL of distilled water. What is the concentration (M) of Cu2+(aq) in the resulting solution (assume that the final volume is 20 mL)?
d). How many mmol of NO3-(aq) are in the resulting solution?
e). You need to prepare 25 ml of 0.25 M nickel perchlorate, Ni(ClO4)2.(aq). The reagent available is Ni(ClO4)2•3H2O.
How many grams of Ni(ClO4)2•3H2O should you weigh out?
f). How many mmol of perchlorate anions, ClO4 - , are in the 25 ml, 0.25 M solution?
g). You also need to prepare a 0.10 M solution of nickel perchlorate. How many mL of the 0.25 M Ni(ClO4)2(aq) should you add to a 10 mL volumetric flask in order to make a 0.10 M solution (after you have added the 0.25 M Ni(ClO4)2, the volumetric flask will be filled to the line with water to give 10 mL of solution)
h). You prepare 0.10 M Rb2S from 0.50 M Rb2S. Only 50 mL of 0.50 M Rb2S is available. What volume (mL) of 0.10 M Rb2S can you make using 50 mL of 0.50 M Rb2S?
Question Set 2
This question set deals with the second part of this tutorial. The preparation and usage of absorbance spectra and calibration graphs, as well as the use of the Beer Lambert Law.
These questions involve using a spectrophotometer to determine the concentration of a ion (M+) aqueous solution. The graphs below show the absorption spectrum for a 0.40 M solution of M+ and a calibration graph
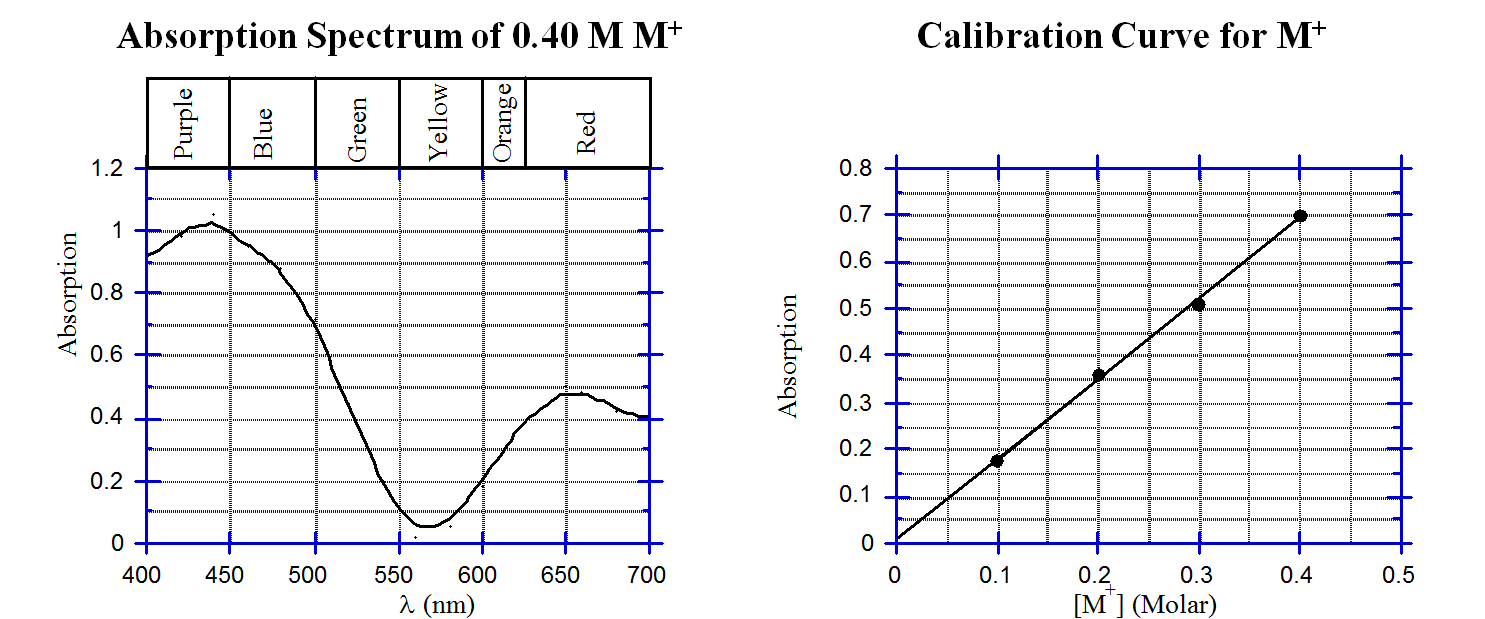
a). What color is the aqueous solution M+?
b). At what wavelength was the calibration graph obtained?
c). A solution M+ is diluted by taking 4.00 mL of M+ solution and adding enough water to give 20.0 mL. The absorbance of the diluted solution is 0.65 at the wavelength that was used to construct the calibration graph (above). What is the molarity of the undiluted sample?
The graph below shows the absorption spectra for an indicator in acidic and basic solutions. In both cases [indicator] = 1.0 x 10-3 M.
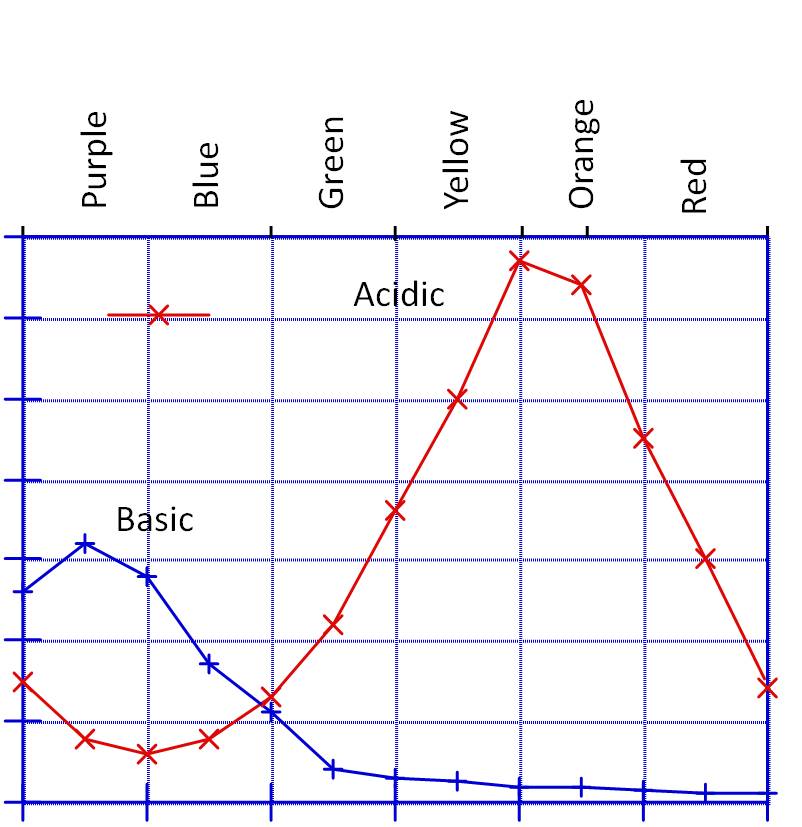
d). What color is this indicator in acid and basic solution?
e). Based on the above specta, which of the following statements are correct?
1. As the solution becomes more basic, % transmission increases at l = 600 nm. (Note that the above graph gives absorbance, not % of transmission.)
2. In acidic solution, the absorption coefficient ( e ) increases as l increases from 500 to 600 mm.
f). An acidic solution of the indicator has an absorbance of 0.25 at l = 575 nm. What is [indicator] in this solution?
