Obtaining an Absorbance Spectrum
Light & Solutions
Most of the light that all of you see, like the light from the sun, is white light. But did you know that the light coming from the sun is made up of many different colors?
It's time you are introduced to Mr Roy G. Biv, the keeper of the colors!!

White light is primarily made up of these seven colors, but the color is different at each wavelength.
You will see the individual colors of each of these wavelengths.
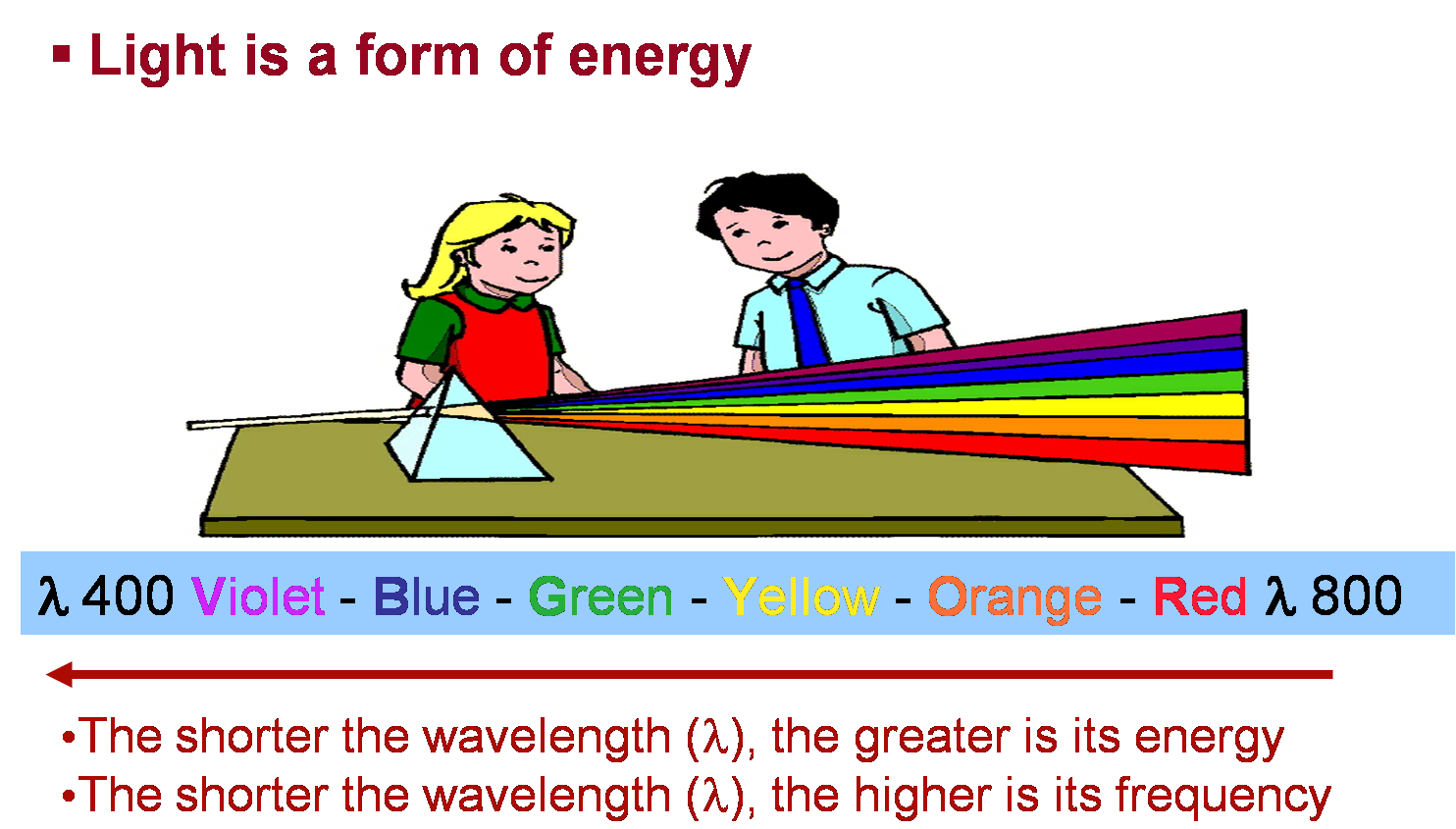
Bright White Light!
When you look at a solution, there is light from all around interacting with it. This is how you are able to see the color that the solution is.
Much of the time, you are seeing how things interact with white light. This light is the light that comes from the sun, and many light bulbs.
It is comprised of all the colors of the rainbow (ROY G. BIV)!!! But what does that have to do with solution color? The color of a solution depends on how the light (of many colors) interacts with the solution, and is a combination of colors from that rainbow!
Below shows how white light would interact with a sample that you have.
1). All the colors of light in white light, hit the solution.
2). Each wavelength of light interacts with the molecules in the solution.
3). Some colors of light are blocked by the molecules and Absorbed, while others pass through and are Transmitted
A question to consider:
If you have a sample of a blue solution, what color light would be the most transmitted?

