Obtaining an Absorbance Spectrum
Laboratory Details
You will take each of the solutions you already made, and create an absorbance spectrum for each one. To do this you will use the spectrophotometer to select a wavelength of light and measure and record the absorbance of the sample at that wavelength.
Use the labeled picture below to guide youself through the steps for taking an absorbance measurement.

1). Tune the spectrophotometer to the desired wavelength
2). Load a cuvette with the solvent used in your sample
What did you use to make your solution? Water? Acid? Base? This is your solvent, and this is what you will use to tare the spectrophotometer.
Make sure the clear sides of the cuvett are in the direction of the light.
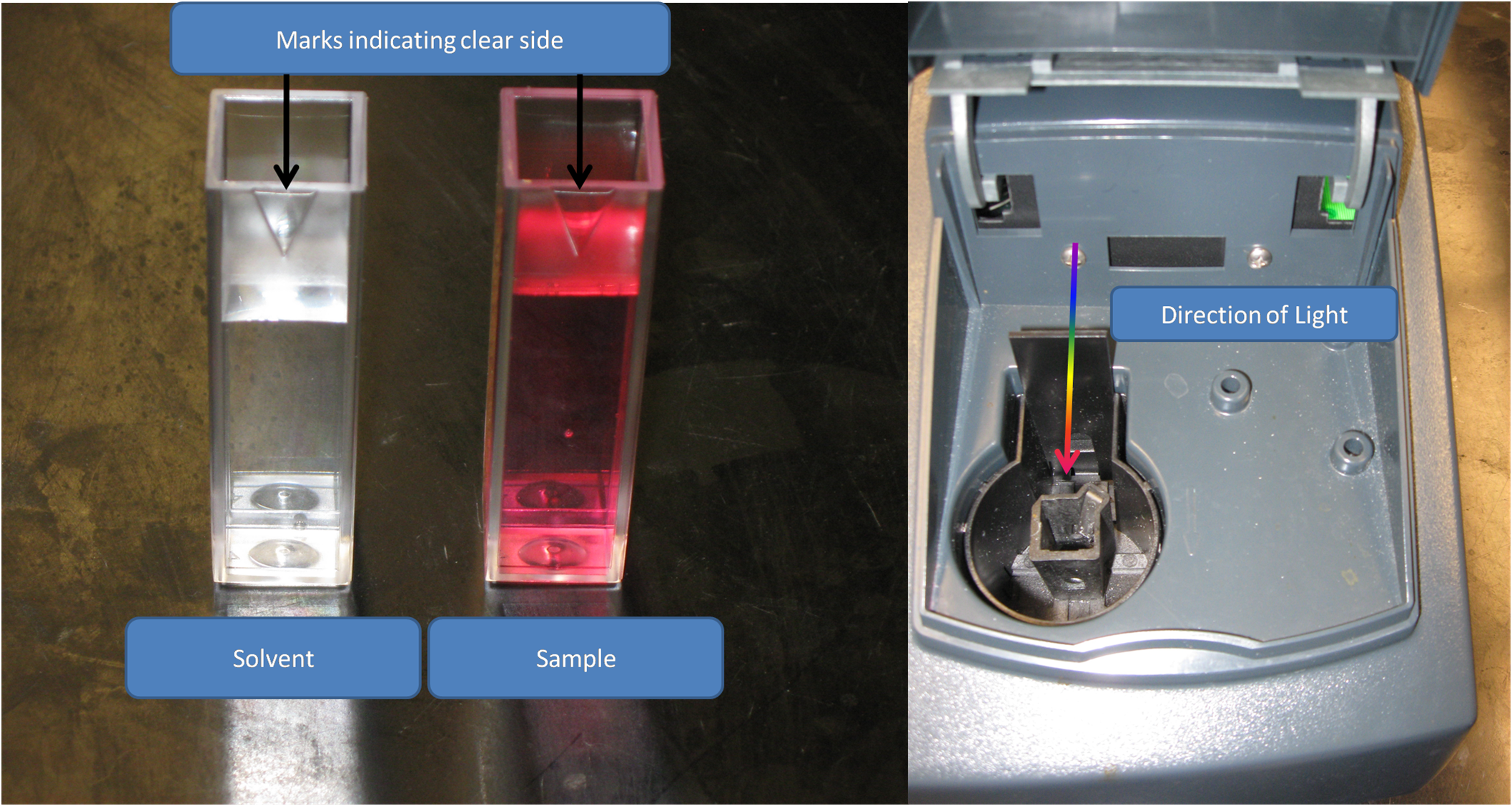
3). Place the cuvette in the spectrophotometer, and close it. Then tare the spectrophotometer.
This is just like taring a balance. You are telling the spectrophotometer that whatever light it is measuring, should be equal to zero absorbance.
4). Once the spectrophotometer is tared, then remove the solvent cuvette, and load a cuvette with your sample. Record the absorbance value that it gives you.
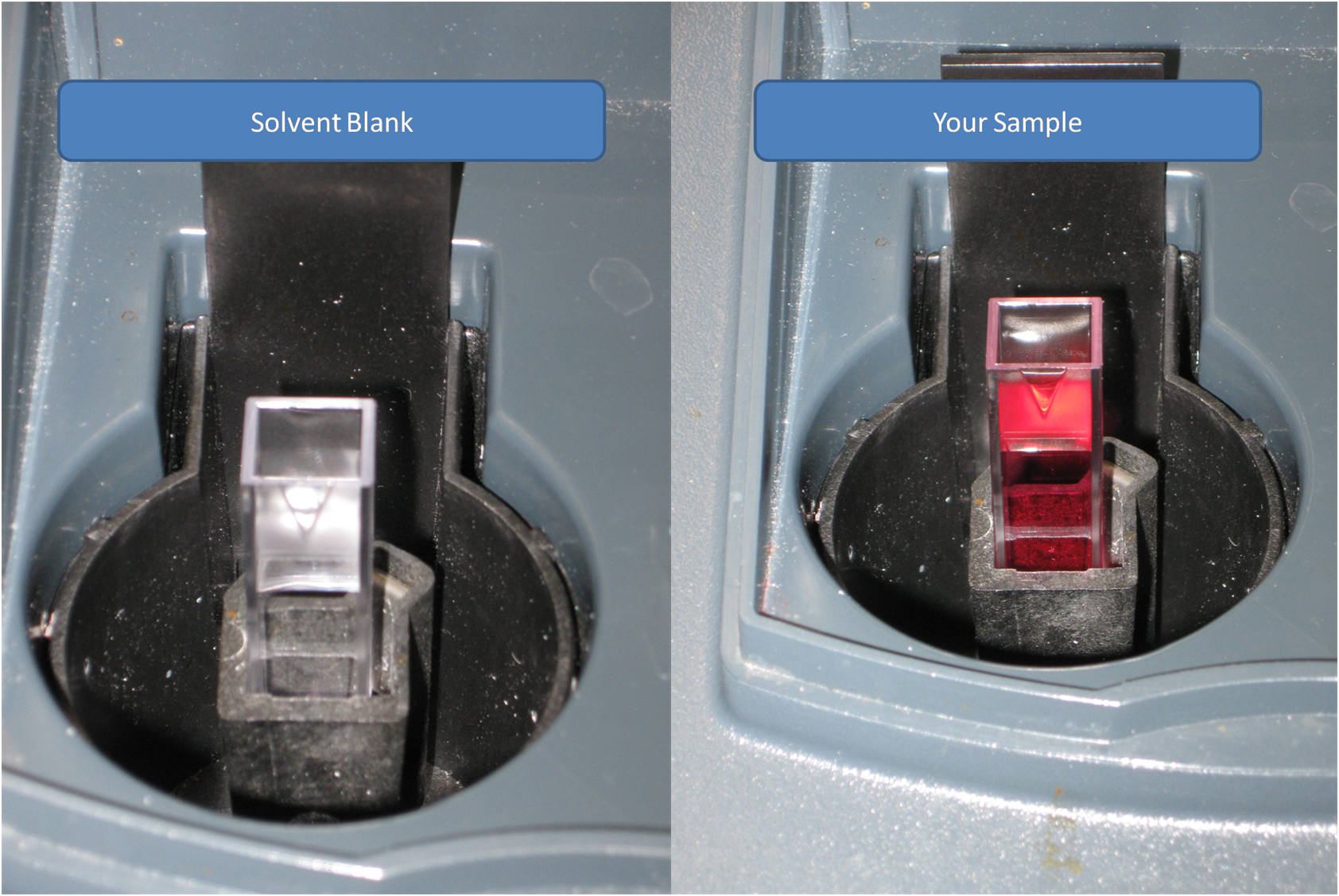
5). This procedure NEEDS to be repeated at EACH wavelength.
Absorbance is different for EVERY chemical at EVERY wavelength, so every time you change one of them, the spectrophotometer will need to be tared
Once you have your data all recorded, then you can plot each of your absorbance spectra
Y-axis will be the absorbance values
X-axis will be the wavelength
Look at the absorbance spectra that you create.
- Look at the maximum absorbance in your graph
- Look at the minimum absorbance in your graph
- What color are your solutions?
 Show/hide comprehension question...
Show/hide comprehension question...
